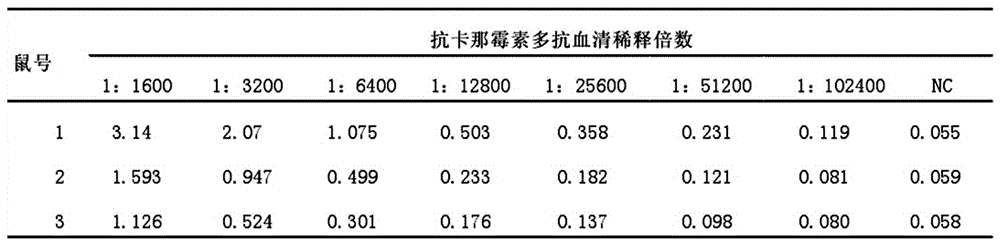
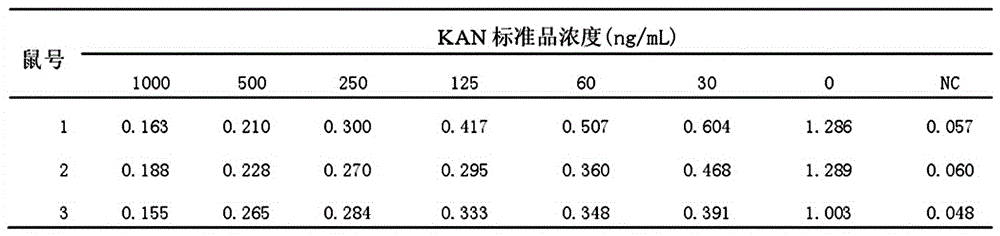
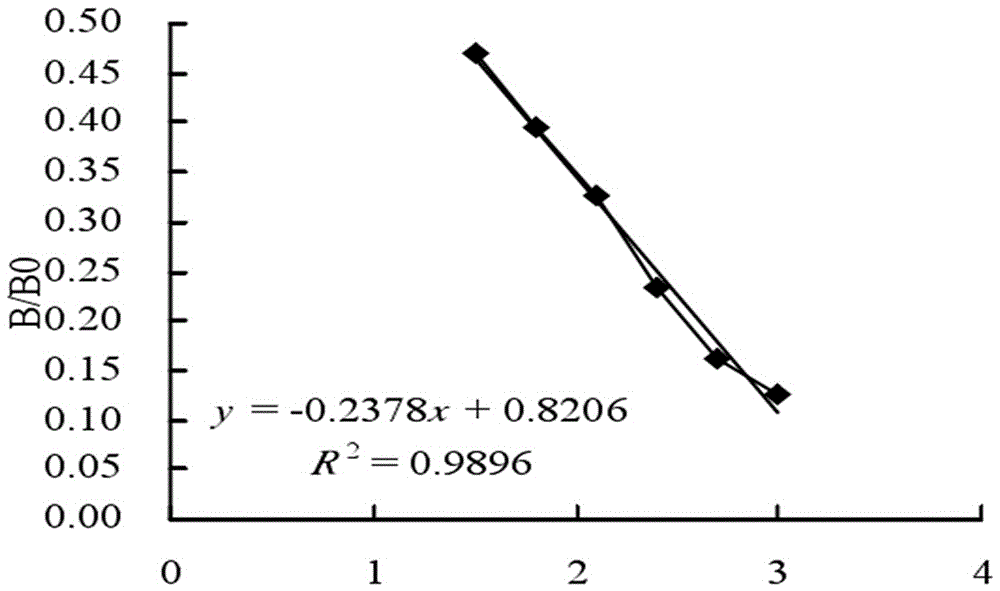
Kanamycin antibody and preparation process thereof
A kanamycin and preparation technology, applied in the field of kanamycin antibody preparation, can solve the problems of poor inhibitory effect on Gram-positive bacteria and achieve strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kanamycin antibody prepared from the following raw materials in parts by weight:
[0027] Kanamycin sulfate 58 parts, bovine serum albumin 5 parts, ovalbumin 3 parts, Freund's complete adjuvant 3 parts, Freund's incomplete adjuvant 3 parts, carbodiimide 3 parts, glutaraldehyde 1 part , 1 part of goat anti-mouse enzyme-labeled secondary antibody, 2 parts of disodium hydrogen phosphate, 8 parts of sodium acetate, 15 parts of sodium dodecylsulfonate, 10 parts of Coomassie brilliant blue R-250, 8 parts of carbamide peroxide, 6 parts of ethanol 5 parts of formaldehyde, 8 parts of sodium borohydride, 1 part of methanol, 1 part of concentrated sulfuric acid, 2 parts of phosphate buffer, 1 part of carbonate buffer CBS for coating, 1 part of washing solution PBST, 5% of blocking solution 1 part of pig serum, 1 part of ELISA test chromogenic solution, 2 parts of ELISA test termination solution.
[0028] The ELISA test chromogenic solution is 0.25 milliliters; the ELISA test te...
Embodiment 2
[0035] A kanamycin antibody prepared from the following raw materials in parts by weight:
[0036] 69 parts of kanamycin sulfate, 15 parts of bovine serum albumin, 4 parts of ovalbumin, 4 parts of Freund's complete adjuvant, 4 parts of Freund's incomplete adjuvant, 4 parts of carbodiimide, 1.5 parts of glutaraldehyde , 2 parts of goat anti-mouse enzyme-labeled secondary antibody, 2.5 parts of disodium hydrogen phosphate, 9 parts of sodium acetate, 17.5 parts of sodium dodecylsulfonate, 12.5 parts of Coomassie brilliant blue R-250, 10 parts of carbamide peroxide, 8.5 parts of ethanol 1 part, 6 parts of formaldehyde, 10.5 parts of sodium borohydride, 2 parts of methanol, 2 parts of concentrated sulfuric acid, 1 part of phosphate buffer, 1 part of carbonate buffer CBS for coating, 1 part of washing solution PBST, and 5% of blocking solution 1 part of serum, 1 part of ELISA test chromogenic solution, 1 part of ELISA test termination solution.
[0037] The ELISA test chromogenic s...
Embodiment 3
[0044] A kanamycin antibody prepared from the following raw materials in parts by weight:
[0045] 80 parts of kanamycin sulfate, 25 parts of bovine serum albumin, 5 parts of ovalbumin, 5 parts of Freund's complete adjuvant, 5 parts of Freund's incomplete adjuvant, 5 parts of carbodiimide, 2 parts of glutaraldehyde , 3 parts of goat anti-mouse enzyme-labeled secondary antibody, 3 parts of disodium hydrogen phosphate, 10 parts of sodium acetate, 20 parts of sodium dodecylsulfonate, 15 parts of Coomassie brilliant blue R-250, 12 parts of carbamide peroxide, 11 parts of ethanol 1 part, 7 parts of formaldehyde, 13 parts of sodium borohydride, 3 parts of methanol, 3 parts of concentrated sulfuric acid, 1 part of phosphate buffer, 1 part of carbonate buffer CBS for coating, 1 part of washing solution PBST, 5% of blocking solution 1 part of pig serum, 1 part of ELISA test chromogenic solution, 3 parts of ELISA test termination solution.
[0046] The ELISA test chromogenic solution i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com