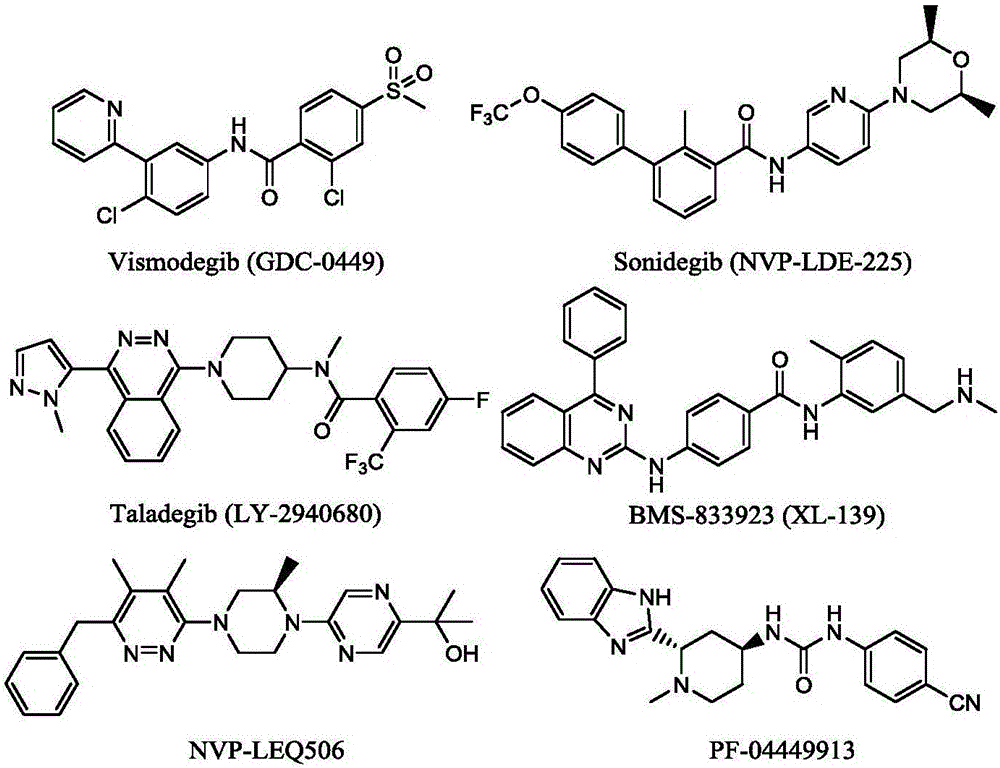
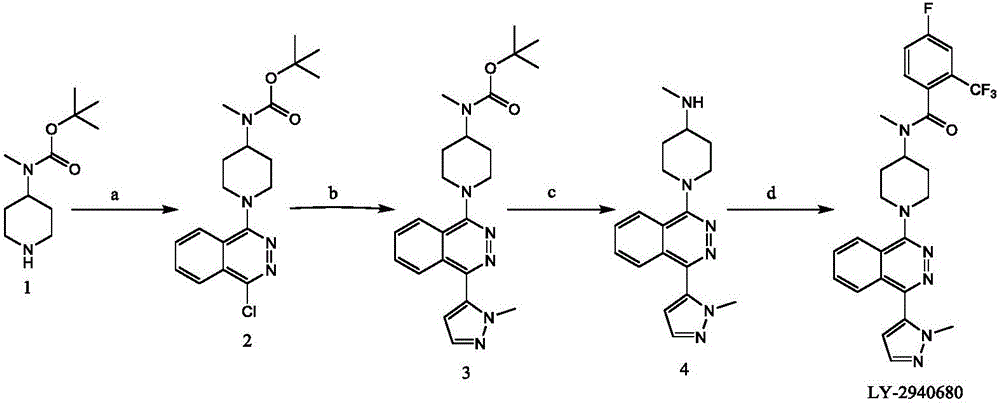
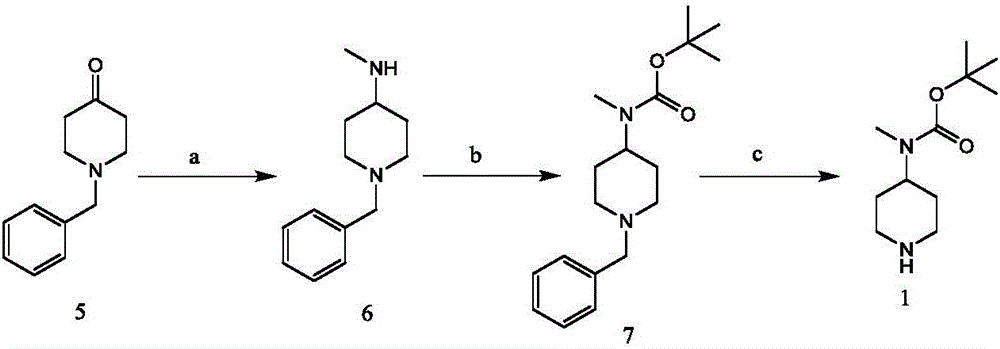
Synthetic method of Taladegib
A synthetic method and compound technology, applied in the field of medicine and chemical industry, can solve the problems of complicated operation, high cost, unsuitable for mass production, etc., and achieve the effect of simple operation and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 compound 6 (structure such as formula 6)
[0039] The raw material N-benzylpiperidone (Formula 5, 2g, 10.568mmol, 1.0eq.) was dissolved in 30mL of methanol, 4 drops of glacial acetic acid was added dropwise to the solution, and after stirring evenly, 0.785g (11.625mmol, 1.1 eq.) of methylamine hydrochloride, react at room temperature (25°C) for 2h; move the reaction into an ice-water bath, and after the reaction temperature drops to 0°C, add 1.328g (21.136mmol, 2eq.) of cyano group in batches Sodium borohydride, the batch addition time is controlled at about 10min; after stirring evenly, remove the reaction from the ice-water bath, and react at room temperature for 16h; after the reaction, pour the reaction mixture into saturated sodium bicarbonate solution or sodium carbonate solution, and use two Chloromethane was extracted three times, the organic layers were combined, dried by adding anhydrous sodium sulfate, and concentrated under re...
Embodiment 2
[0042] The preparation of embodiment 2 compound 8 (structure such as formula 8)
preparation example 2-1
[0043] Preparation Example 2-1: Dissolve compound 6 (1.5g, 7.342mmol, 1eq.) in 30mL of dichloromethane, add 2mL of triethylamine to the solution, stir well and add 1.663g (1eq.) of 4-fluoro-2-(trifluoromethyl)benzoyl chloride (CAS No. 189807-21-4), reacted at room temperature for 6h; after the reaction, concentrated under reduced pressure to obtain a crude product, purified by column chromatography (eluent (dichloromethane / methanol, the volume ratio is 20:1) to obtain the pure product 8 (compound 8, 2.720 g, yield 94%) as a colorless oil.
[0044] The test data of product 8 are as follows:
[0045] 1 H NMR (300Mz, CDCl 3 )δ7.39(m,1H),7.32-7.22(m,7H),4.60(m,1H),3.40(s,2H),3.10-2.83(m,2H),2.65(s,3H),2.16 (m,2H),1.93-1.58(m,4H)ppm; MS(ESI)m / z:[M+H] + = 395.18077.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com