Tumor-targeting lipophilic positive ion-chlorambucil compound and preparation method and application to albumin nano-drug
A tumor-targeting, chlorambucil technology, applied in the field of biomedicine, can solve the problems of low selectivity and multi-drug resistance of anti-cancer drugs, and achieve the advantages of drug delivery, prolonged in vivo imaging, and enhanced fluorescence. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
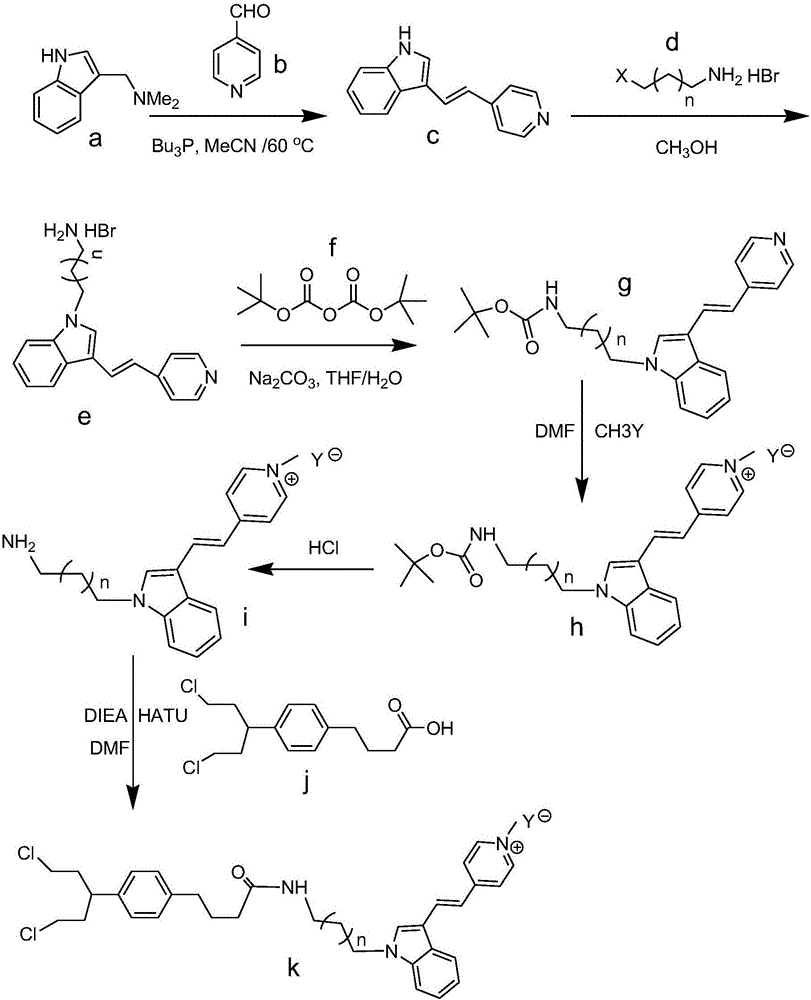
[0051] First of all, take compound 1 trophylline with compound 2 pyridinecarbaldehyde The reaction gives compound 3, the structure of which is shown below:
[0052]
[0053] During the specific implementation, in the reaction bottle, add agrophylline (about 1.8g, 10.0mmol), pyridine formaldehyde (2.0eq), tributylphosphine (2.0eq) and anhydrous acetonitrile 50ml, then heat and reflux at 60°C overnight . After the reaction is complete, cool the reaction system to room temperature, remove the organic solvent under reduced pressure, concentrate to obtain an oil, and pass through a silica gel chromatography column (n-hexane / ethyl acetate, the volume ratio is (1~4):1, preferably 1:1) After purification, 1.9 g of compound 3 was obtained.
[0054] Next, add compound 3 (1.0g, 4.5mmol), 3-bromopropylamine hydrobromide in the reaction flask (1.0eq), 10ml of anhydrous methanol (or anhydrous ethanol, anhydrous ether), and then heated and refluxed overnight; the reaction was compl...
Embodiment 2
[0067] The method for preparing compound 3 is the same as that in Example 1, and will not be repeated here.
[0068] Take compound 3 to react with compound 10,
[0069]
[0070] Add compound 3 (1.0g, 4.5mmol), compound 10 (1.0eq), anhydrous methanol (or absolute ethanol, anhydrous ether) 10ml in the reaction flask, then heat and reflux to react overnight; the reaction is complete, and the reaction system is cooled to Room temperature, filtered, washed with a small amount of methanol, and dried to obtain light red semi-solid compound 11 (1.37g); in this example, the structure of compound 11 is as follows:
[0071]
[0072] Next, add compound 11 (1.0g, 2.5mmol), 20ml of tetrahydrofuran, 20ml of anhydrous sodium carbonate (2.0eq) in water, stir at room temperature for 30min, and then add di-tert-butyl dicarbonate (1.1eq) dropwise Dissolve in 10ml of tetrahydrofuran, react at 25-30°C for 6 hours; filter, spin the filtrate to dryness, then add 30ml of dichloromethane and met...
Embodiment 3
[0080] Example 3: Preparation and identification of conjugated compound albumin nanomedicine
[0081] For the coupling compounds 9 (F3CBL) and 15 (F6CBL) prepared in the foregoing Example 1 and Example 2, they were dissolved in a small amount of DMSO, and then dropped into the stirred human serum albumin phosphate buffer (pH: 7.4, the protein concentration is 0.5-20 mg / ml), stirred at 4-10°C for 10-120 min at low temperature, dialyzed in PBS overnight in a dialysis bag (molecular weight > 8000), and the albumin nanomedicine coupled with compound 9 or 15 can be obtained.
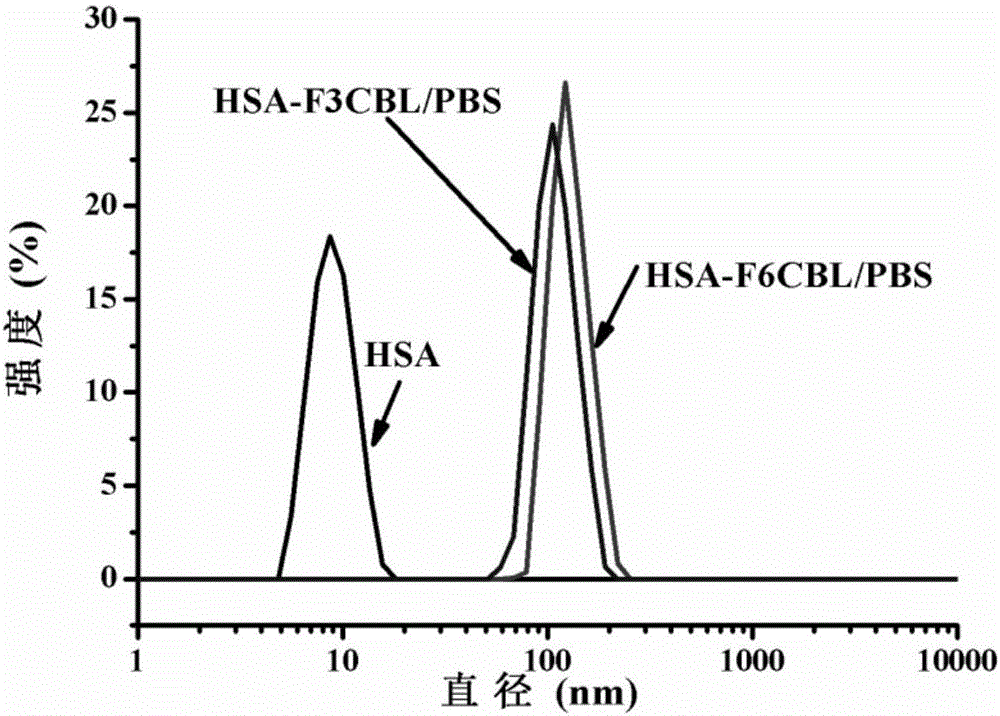
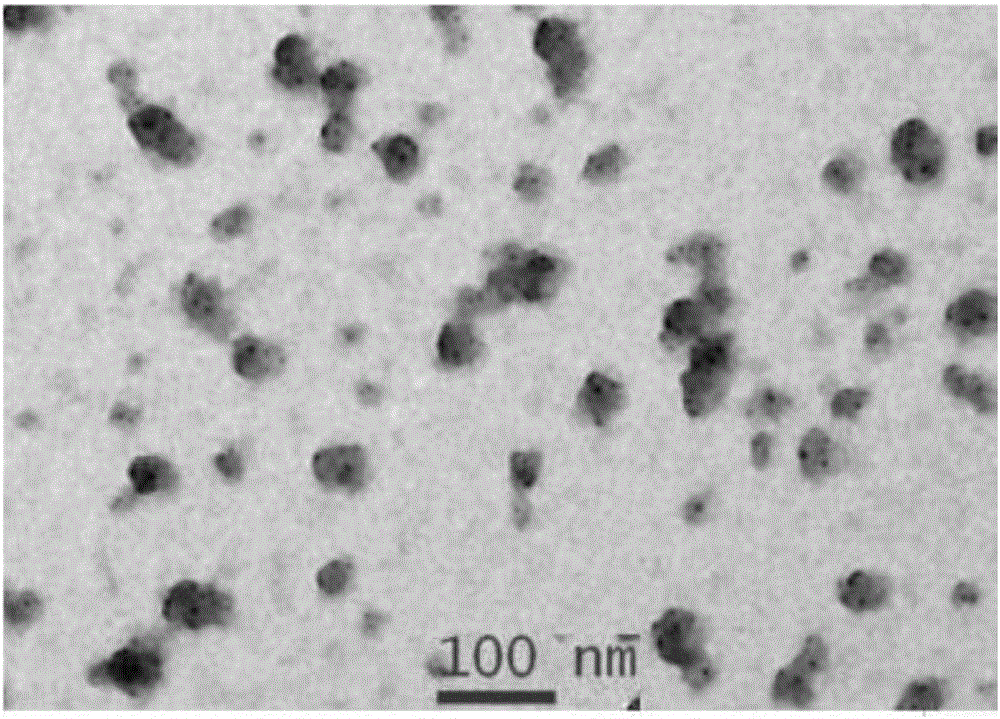
[0082] The albumin nano-medicines of compounds 9 and 15 and human serum albumin (HSA) were subjected to dynamic light scattering (Dynamic Light Scattering) DLS to measure the hydrated particle size of albumin particles in nanometer size. From the DLS analysis results, The hydrated particle sizes of compounds 8 and 14 are about 105nm and 120nm, and the dispersibility is good, and the PDI value is figure 2 . ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com