Palladium-ion multi-channel response probe and synthesis method and application thereof
A synthesis method and technology of palladium ions, which are applied in the direction of chemical reaction of materials, material analysis by observing the influence of chemical indicators, and material analysis by optical means, which can solve problems such as few researches.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of Rhodamine Glide Hydrazide
[0055] In a 100mL two-necked flask equipped with a stirring magnet and a spherical condenser, add rhodamine B (1.000g, 2.10mmol), ethanol (20mL) and hydrazine hydrate (80%, 0.50mL, 8.60mmol) successively, and stir Heat under reflux until the reaction solution turns from dark red to light yellow, and the reaction temperature is 80°C, stop heating, and cool to room temperature. The solvent was distilled off under reduced pressure, and the residue was separated by column chromatography (ethyl acetate / petroleum ether=1:1, R f =0.40), 0.800 g of light pink solid was obtained, the yield was 83%, m.p.: 179-181°C.
Embodiment 2
[0057] Synthesis of Ferrocene-Rhodamine Probe
[0058] In a 50mL two-necked flask equipped with a stirring magnet and a spherical condenser, add rhodamine lactam (0.100g, 0.22mmol), ferrocene formaldehyde (0.050g, 0.22mmol), ethanol (3.0mL) and ice Acetic acid (0.01mL), the mixed solution was stirred and heated to reflux, the reaction temperature was 80°C, followed by thin layer chromatography, after the reaction was complete, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (ethyl acetate / petroleum ether = 1:1, R f =0.30), to obtain 0.120 g of an orange powdery solid, with a yield of 85%, m.p.: 165-167° C. 1 H NMR (300MHz, CDCl 3 ,TMS):δ8.29(s,1H),8.00(d,1H,J=6.0Hz),7.49(m,2H),7.12(d,1H,J=6.0Hz),6.60(s,2H) ,6.49(d,2H,J=3.0Hz),6.30(d,2H,J=3.0Hz),4.51(s,2H),4.24(s,2H),3.86(s,5H),3.35(q, 8H, J=7.0Hz), 1.16(t, 12H, J=7.0Hz). 13 C NMR (75MHz, CDCl 3 ,TMS): δ164.27,153.06,151.72,148.95,148.68,132.94,129.62,128.13,123.64...
Embodiment 3
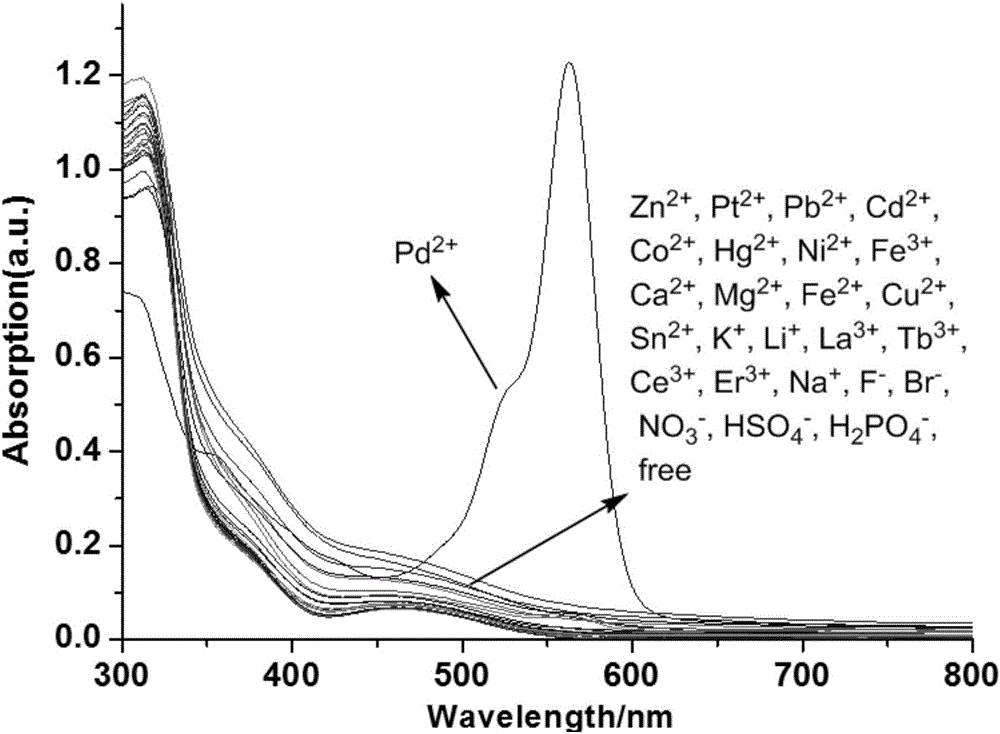
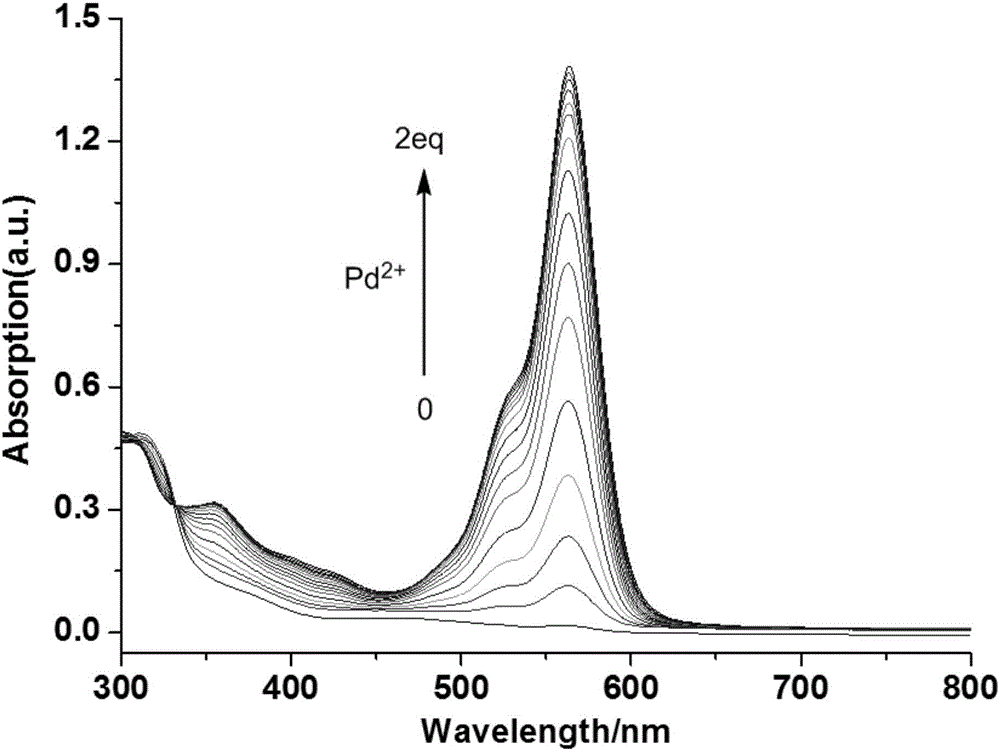
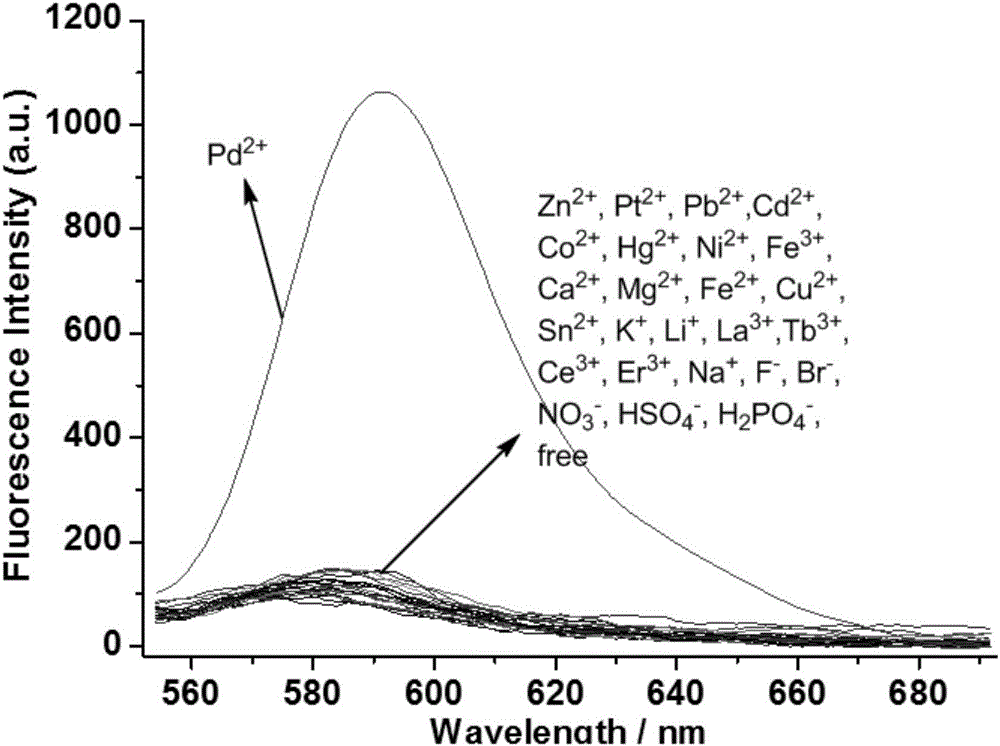
[0060] Ultraviolet Spectrum Detecting the Effects of Probes on Various Metal Ions
[0061] Prepare 1.25×10 in a 50mL volumetric flask -4 Probe molecule THF solution in the embodiment 2 of M, prepare 2.5 * 10 respectively in 10mL volumetric flask -2 Aqueous solutions of different metal ions of M; respectively pipette 2.0mL1.25×10 in a 10mL volumetric flask - 4 The probe molecule THF solution of M and 20 μL of the above-mentioned metal ion aqueous solution were fixed to volume with deionized water, and their ultraviolet absorption spectra were measured respectively ( figure 1 ).
[0062] The results show that only adding Pd 2+ ion, the ultraviolet spectrum absorption of the probe molecule changes significantly, and a new strong ultraviolet absorption peak appears at 560nm, indicating that the probe molecule can be highly selective with Pd 2+ Ionic coordination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com