Synthetic method of benzofuran-2(3H)-one
A technology of benzofuran and synthesis method, which is applied in the field of synthesis of organic compounds, can solve the problems of lye waste water consumption, loss of products, increase of production cost, etc., and achieve the effects of reducing the generation of three wastes, avoiding material loss, and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
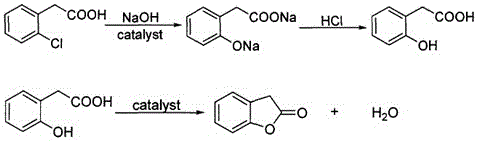
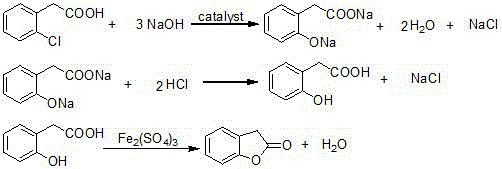
[0028] (1) Add 139.3g of o-chlorophenylacetic acid (98%, 0.8mol), 112.0g of sodium hydroxide, 261.3g of water and 0.8g of copper oxide into a 500mL autoclave, and stir to raise the temperature to 200°C. Under the condition of heat preservation and reaction for 12 hours, HPLC analysis showed that the content of o-chlorophenylacetic acid was 0.81%. After cooling down to room temperature, open the autoclave, pour the reaction solution into a beaker, adjust the pH to 1-2 with hydrochloric acid, cool down to 10°C, and pump Filter and dry to obtain 123.3 g of o-hydroxyphenylacetic acid. The purity of the product by HPLC analysis is 90.0%, and the yield is 91.2% (calculated as o-chlorophenylacetic acid).
[0029] (2) Add 50.7g (90%, 0.3mol) of o-hydroxyphenylacetic acid obtained in step (1), 235mL of toluene and 0.5g of anhydrous ferric sulfate into a 500mL reaction flask, stir and heat to reflux for reaction, and collect in the water separator The water generated by the reaction, to...
Embodiment 2
[0031] (1) Add 139.3g of o-chlorophenylacetic acid (98%, 0.8mol), 112.0g of sodium hydroxide, 261.3g of water and 0.8g of copper oxide into a 500mL autoclave, and stir to raise the temperature to 200°C. Under the condition of heat preservation for 12 hours, HPLC analysis showed that the content of o-chlorophenylacetic acid was 0.50%. After cooling down to room temperature, open the autoclave, pour the reaction solution into a beaker, adjust the pH to 1-2 with hydrochloric acid, cool down to 10°C, and pump Filter and dry to obtain 122.5 g of o-hydroxyphenylacetic acid. The purity of the product by HPLC analysis was 89.5%, and the yield was 90.1% (calculated as o-chlorophenylacetic acid).
[0032] (2) Add 51.0g (89.5%, 0.3mol) of o-hydroxyphenylacetic acid obtained in step (1), 250mL of toluene and 0.5g of anhydrous ferric sulfate into a 500mL reaction flask, stir, heat and reflux for reaction, and collect in a water separator The water generated by the reaction and toluene were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com