Annonaceous acetogenins nanoparticles taking cyclodextrin and lecithin as vectors as well as preparation method and application of annonaceous acetogenins nanoparticles
A cyclodextrin and nanoparticle technology, which can be applied to non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Low problems, to achieve significant anti-tumor efficacy, simple process, no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
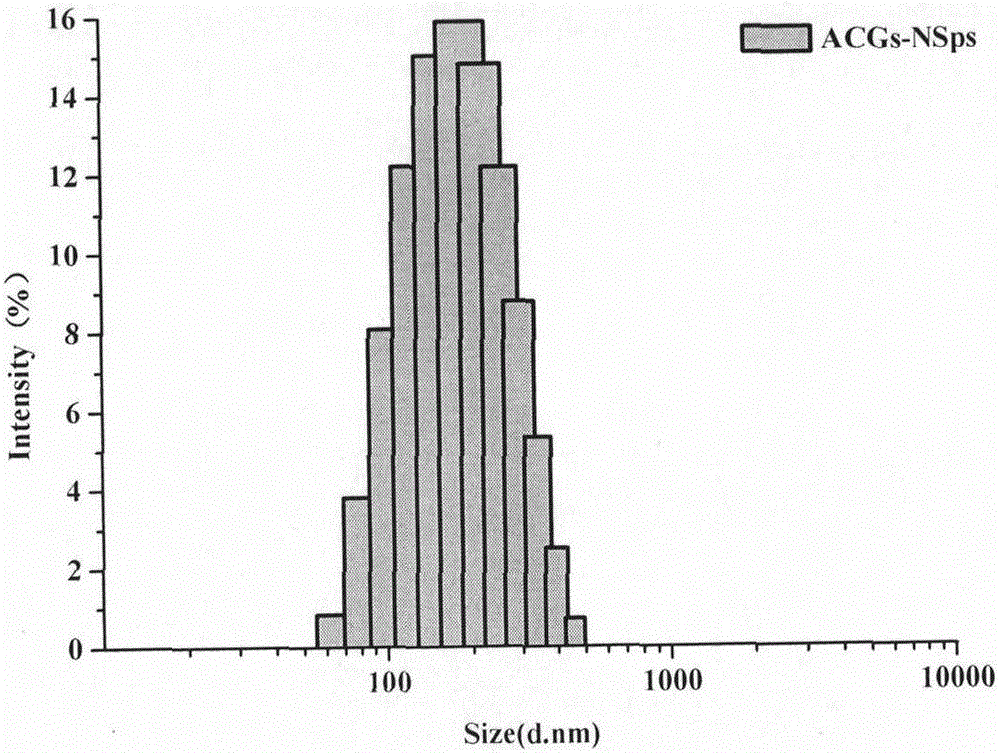
[0048]Weigh 2 mg of soybean lecithin and dissolve it in 0.2 mL of methanol, slowly inject it into 4 mL of aqueous solution containing 4 mg of hydroxypropyl-β-cyclodextrin at 25°C and 500 rpm under stirring conditions, continue stirring for 10 minutes, and then rotary evaporate to remove methanol. Subsequently, 8 mg of ACGs was dissolved in 0.4 mL of methanol, injected into the above-obtained solution at room temperature and stirred at 500 rpm, and then the methanol was removed by rotary evaporation to obtain ACGs nanoparticles. The average particle size is 144.4nm ( figure 1 ), the polydispersity index (PDI) was 0.08, and the potential value was -22.9mV.
Embodiment 2
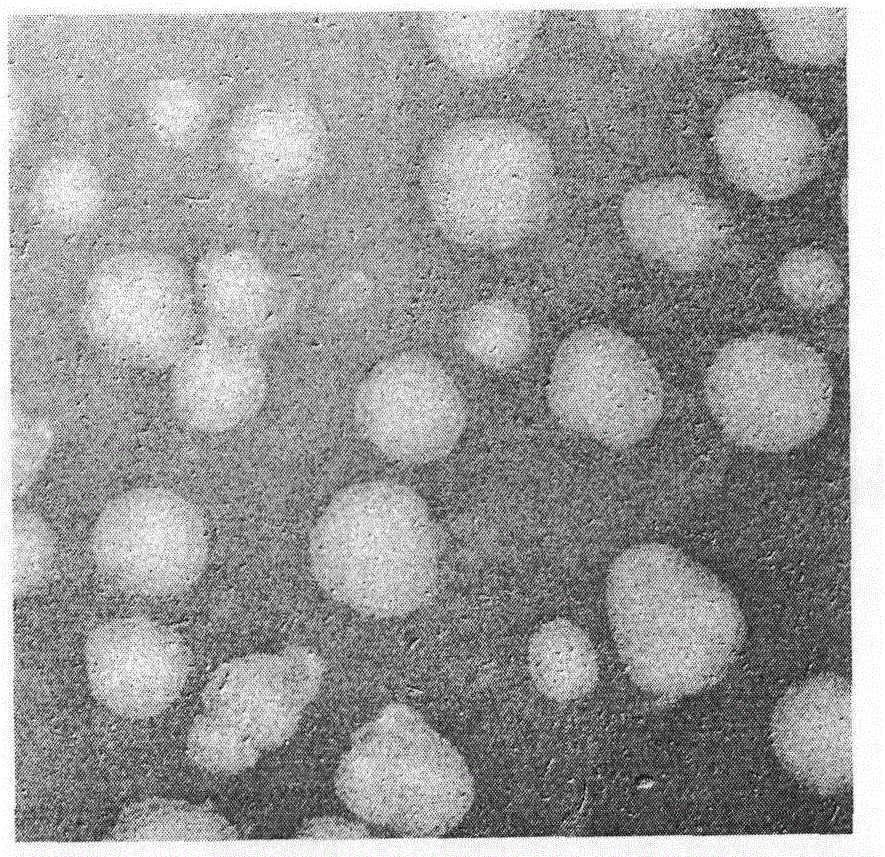
[0050] Prepare ACGs suspension at a concentration of 2 mg / mL, absorb 6 μL and drop it on a 300-mesh copper grid, let it dry naturally in the air, and then stain it with 0.1% uranyl acetate for 10 min, observe the morphology of the particles under a transmission electron microscope ( figure 2 ).
Embodiment 3
[0051] Example 3 Investigation of the Stability of ACGs Nanoparticles in Artificial Gastrointestinal Fluid
[0052] Preparation of artificial gastric juice: Take 16.4mL of dilute hydrochloric acid with a concentration of 1mol / L, add 800mL of distilled water, 10g of pepsin, mix well, add water to dilute to 1000mL.
[0053] Preparation of artificial intestinal juice: 6.8g potassium dihydrogen phosphate, add 500mL water, adjust pH to 6.8 with 0.1mol / L sodium hydroxide, take another 10g trypsin, add water to dissolve, mix the two liquids and add water to dilute to 1000mL.
[0054] Take 0.5mL of the prepared artificial gastrointestinal fluid after passing through the membrane, mix it with ACGs nanoparticles in equal volume, and measure the change of its particle size at a certain time point.
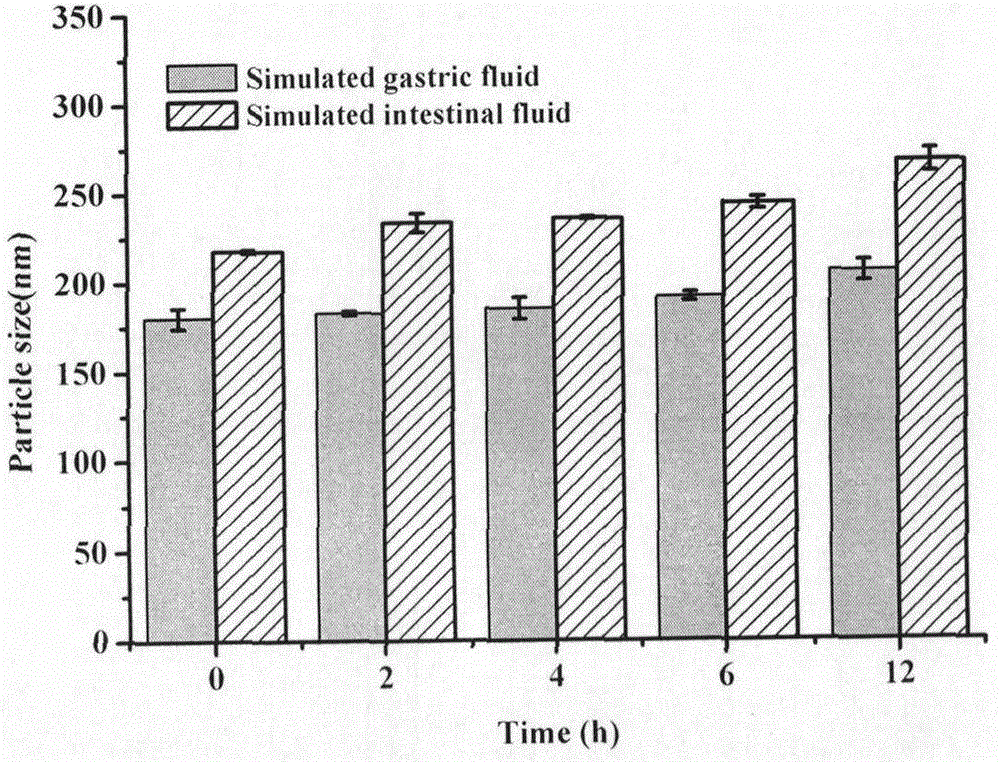
[0055] Results: In the artificial gastrointestinal fluid, the particle size of ACGs nanoparticles changed little within 12 hours ( image 3 ), indicating that ACGs nanoparticles are basicall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Potential value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com