Environmental response type cationic polymer for active dye salt-free dyeing and preparation method thereof
A cationic polymer, salt-free dyeing technology, applied in the field of textile chemistry and dyeing and finishing engineering, to achieve high commercialization potential, reduced sensitivity, and the effect of solving uneven dyeing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
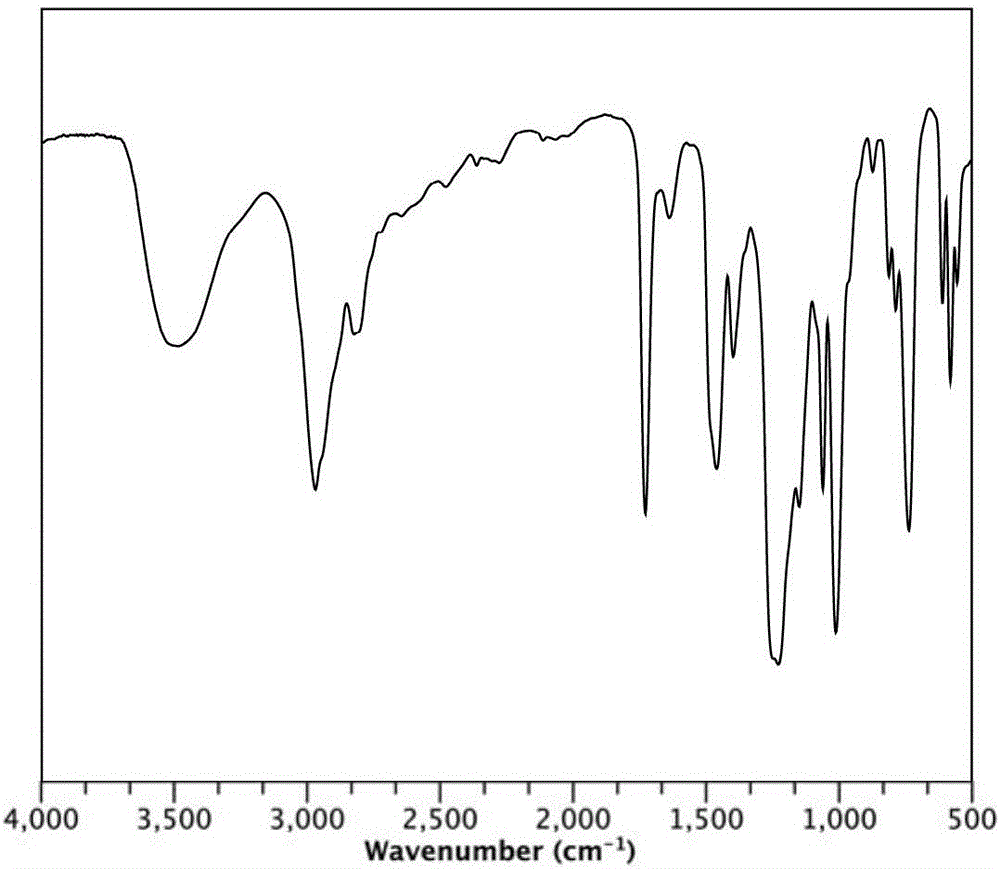
Image
Examples
Embodiment 1
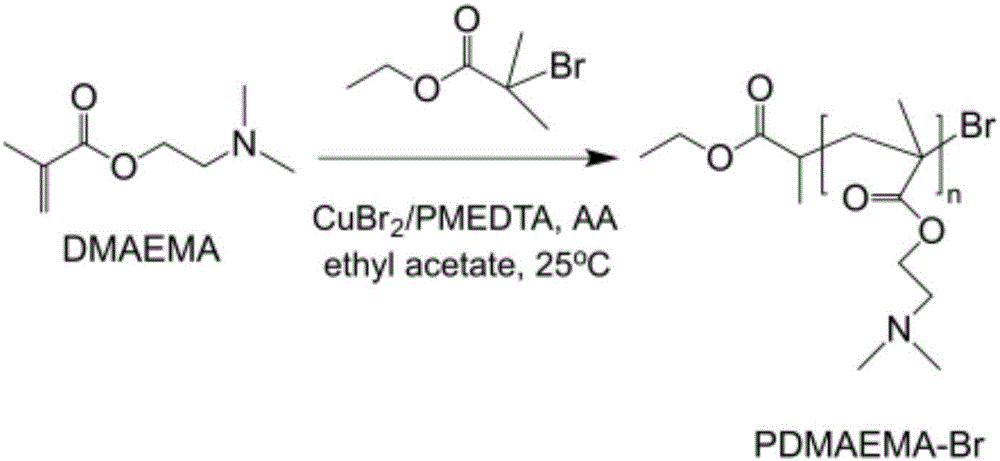
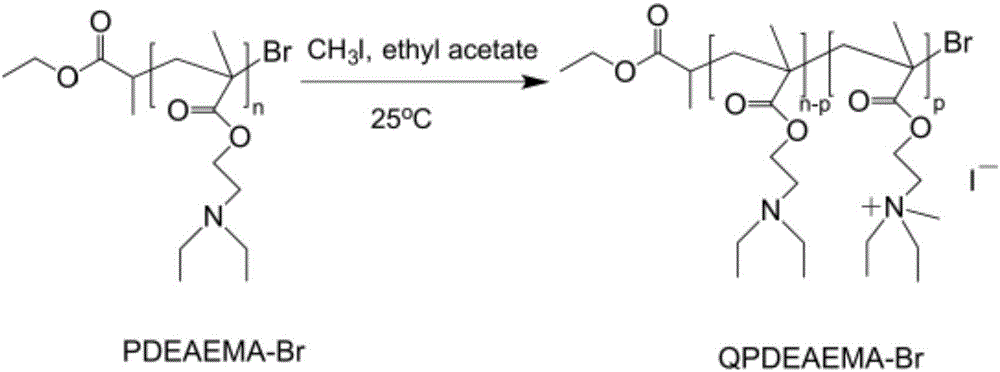
[0034] Glucose (0.05mmol) was dissolved in 10ml ethyl acetate at room temperature, and monomer DEAEMA (25mmol), catalyst CuBr 2 (0.05mmol) and complexing agent PMDETA (1mmol) were added to a 100mL reaction eggplant bottle in turn, and the initiator ECP (64μL) was added under nitrogen protection. Magnetic stirring to make it fully mixed, repeated freezing and vacuuming three times, passing nitrogen gas, reacting in an oil bath at 25°C for 60 minutes, taking out the reaction eggplant bottle and adding ethyl acetate to dilute, then passing the reaction solution in the bottle through a neutral alumina column, The Cu complex in the reaction system was removed, and the filtrate was distilled under reduced pressure to remove the ethyl acetate solvent, precipitated in cold petroleum ether, and dried in vacuum to constant weight to obtain a colorless and transparent polymer product PDEAEMA polymer, which was dissolved in an appropriate amount of Freeze-dry in 1,4-dioxane for later use....
Embodiment 2
[0037] Take ascorbic acid (0.05mmol) and dissolve in 20ml tetrahydrofuran at room temperature, then monomer DMAEMA (25mmol), catalyst CuCl 2 (0.05mmol), complexing agent PMDETA (0.8mmol) were sequentially added to a 100mL reaction eggplant bottle, and the initiator EBiB (64μL) was added under nitrogen protection. Magnetic stirring to make it fully mixed, repeated freezing and vacuuming three times, passing nitrogen gas, reacting in an oil bath at 100°C for 60 minutes, taking out the reaction eggplant bottle and adding tetrahydrofuran to dilute, passing the reaction solution in the bottle through a neutral alumina column to remove the reaction solution. Cu complex in the system, the filtrate was distilled under reduced pressure to remove the tetrahydrofuran solvent, precipitated in cold petroleum ether, and dried in vacuum to constant weight to obtain a colorless and transparent polymer product, which was dissolved in an appropriate amount of 1,4-dioxane Freeze dry in a ring fo...
Embodiment 3
[0040] Get initiator p-TsCl (64 μ L) to be dissolved in 10ml ethyl acetate at room temperature, then add reducing agent AA (0.05mmol) successively to reaction eggplant bottle, complexing agent PMDETA (1.2mmol), solvent ethyl acetate ( 10ml), monomer DMAEMA (25mmol) and catalyst CuCl 2 (0.5 mmol). Magnetic stirring to make it fully mixed, repeated freezing and vacuuming twice, passing nitrogen gas, reacting in an oil bath at 60°C for 60 minutes, taking out the reaction eggplant bottle and exposing it to air to terminate the reaction, passing the reaction solution in the bottle through a neutral alumina column , to remove the Cu complex in the reaction system, the filtrate was distilled under reduced pressure to remove the organic solvent, precipitated in cold petroleum ether, and dried in vacuo to constant weight to obtain a colorless transparent polymer product, which was dissolved in an appropriate amount of 1,4- Freeze-dry in dioxane for later use.
[0041] Add 1g of PDMAE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com