Method for detecting content of sodium, potassium and chloride ions in polyethylene glycol electrolyte preparation
A technology of chloride ion content and determination method, which is applied in the direction of measuring devices, material separation, instruments, etc., can solve problems such as errors in titration end point judgment, different anion and cation determination methods, and risks of preparation quality control, and achieve deviations in test results Small size, high sample detection efficiency, and the effect of saving detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
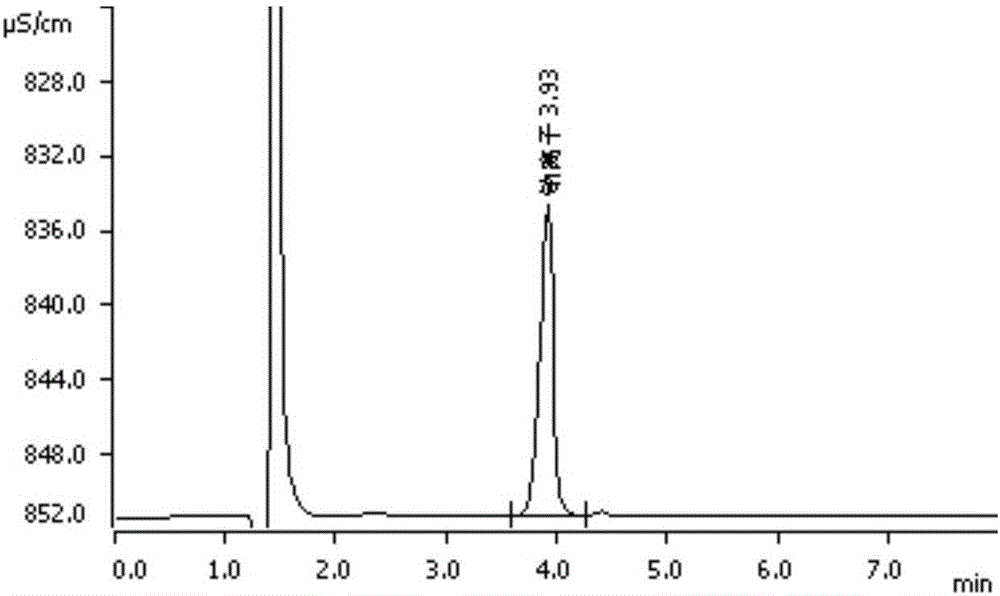
[0044] Embodiment 1: external standard method measures sodium, potassium, chloride ion content in polyethylene glycol electrolyte powder
[0045] 1. Chromatographic conditions:
[0046] Instrument: Wantong 940 ion chromatograph;
[0047] Chromatographic column: ion exchange column, cation column 4.6×150mm, anion column 4.6×100mm;
[0048] Detector: conductivity detector;
[0049] Eluent: cationic 2.5mM nitric acid aqueous solution, anionic 3.5mM sodium carbonate and 1.0mM sodium bicarbonate aqueous solution
[0050] Flow rate: cation 1.0ml / min, anion 0.7ml / min;
[0051] Column temperature: 40°C;
[0052] Injection volume: 20 μl.
[0053] 2. Experimental steps:
[0054] 1. Determination of control and other maps
[0055]Take standard solutions of sodium, potassium, and chloride ions (purchased from China Institute of Metrology) with known contents respectively, and prepare solutions containing sodium 10 μg / ml, potassium 1.25 μg / ml, and chlorine 12.5 μg / ml with water as t...
Embodiment 2
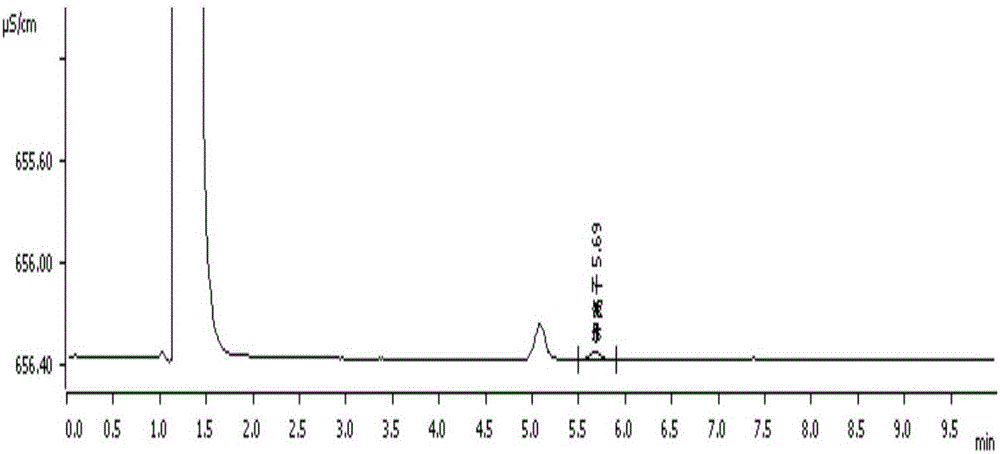
[0082] Embodiment 2: external standard method measures sodium, potassium, chloride ion content in polyethylene glycol electrolyte oral liquid
[0083] 1. Chromatographic conditions:
[0084] Instrument: Wantong 940 ion chromatograph;
[0085] Chromatographic column: ion exchange column, cation column 4.6×150mm, anion column 4.6×100mm;
[0086] Detector: conductivity detector;
[0087] Eluent: cationic 2.3mM nitric acid aqueous solution, anionic 3.2mM sodium carbonate and 1.1mM sodium bicarbonate aqueous solution
[0088] Flow rate: cation 1.2ml / min, anion 0.8ml / min;
[0089] Column temperature: 35°C; injection volume: 20 μl.
[0090] 2. Experimental steps:
[0091] 1. Determination of control spectrum
[0092] The experimental procedure is the same as in Example 1. Anion and cation eluents have no interference to the detection of each ion content, polyethylene glycol electrolyte oral liquid excipients (i.e. polyethylene glycol, acesulfame potassium, sucralose, methylpar...
Embodiment 3
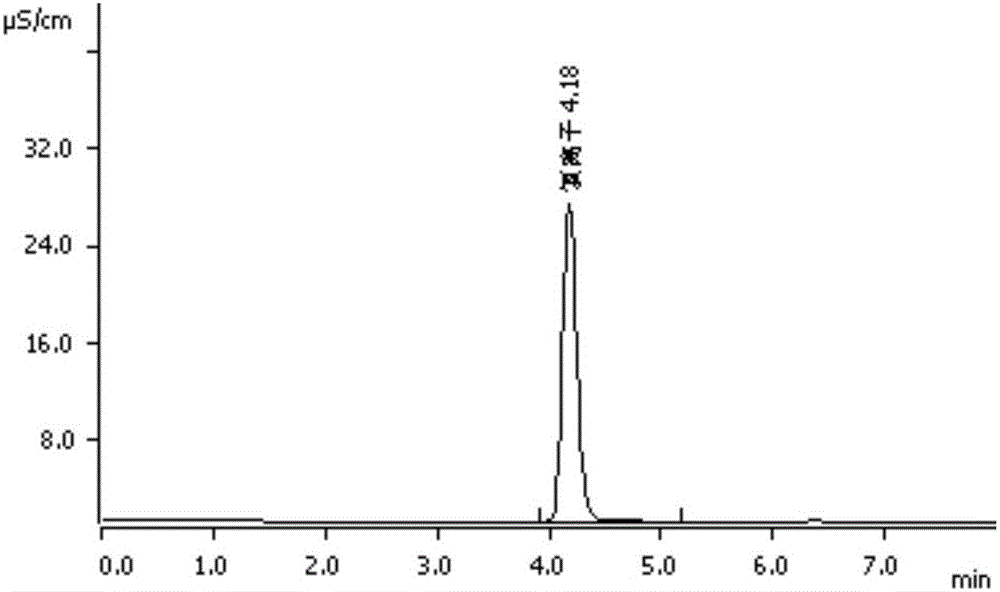
[0111] Embodiment 3: external standard method measures sodium, potassium, chloride ion content in polyethylene glycol electrolyte oral liquid
[0112] 1. Chromatographic conditions:
[0113] Instrument: Wantong 940 ion chromatograph;
[0114] Chromatographic column: ion exchange column, cation column 4.6×150mm, anion column 4.6×100mm;
[0115] Detector: conductivity detector;
[0116] Eluent: cationic 2.7mM nitric acid aqueous solution, anionic 3.8mM sodium carbonate and 0.9mM sodium bicarbonate aqueous solution
[0117] Flow rate: cation 0.8ml / min, anion 0.6ml / min;
[0118] Column temperature: 45°C;
[0119] Injection volume: 20 μl.
[0120] 2. Experimental steps:
[0121]Experimental procedure is with embodiment 2, detects the chromatogram that obtains according to above-mentioned detection condition, as Figure 13 . The measured sodium, potassium, and chloride ion content results are shown in Table 8, and the inspection results such as symmetry factors are shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com