Preparation method of chiral aminoheterocyclic compound and derivative thereof
A technology for aminoheterocycles and compounds, applied in the field of preparation of chiral aminoheterocycles and their derivatives, capable of solving the limitations of large-scale industrial production of 3-aminopyrrolidine and its derivatives, the starting material R-3 - tert-butoxycarbonylpyrrolidine is expensive, restricts mass production and application, and achieves short reaction time, simple and easy method, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Transaminases from different sources catalyze the transamination of N-Cbz-3-pyrrolidone to generate chiral benzyloxycarbonyl-3-aminopyrrolidine
[0068] Add 0.2g substrate N-Cbz-3-pyrrolidone to a 50ml reaction bottle, add 2mgPLP and 10wt crude enzyme solution of corresponding transaminase (see Table 1) in Table 1, 900mg glucose, 290mgD / L-alanine, 20mg coenzyme Glucose dehydrogenase, 20mg coenzyme formate dehydrogenase, system pH=8.0, reaction temperature 30°C, react with 200rpm for 40h; use 10N NaOH to adjust pH=10 to terminate the reaction, extract with 10ml dichloromethane, centrifuge at 12000rpm for 5min, The reaction conversion rate and product chirality were detected by organic phase HPLC. The experimental results are shown in Table 1.
[0069] Table 1 Source and catalytic result of transaminase
[0070]
[0071]
[0072]
[0073] "-" means not detected.
[0074] According to the results in Table 1, it can be known that transaminase 1 (deriv...
Embodiment 2
[0075] Example 2: Cloning and expression of transaminase recombinant strains
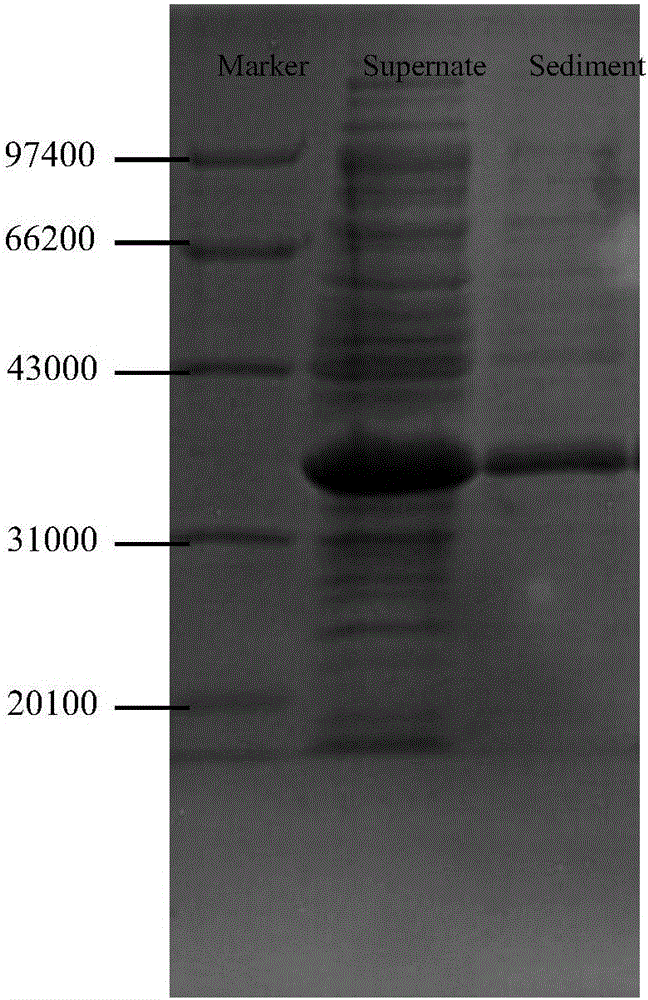
[0076] The transaminase 1 gene containing compatible restriction enzyme sites was double-digested with NdeI and XhoI, and the expression vector pET-22b (+) was double-digested at the same time (purchased from Novagen, product number 69744 ), the digested target gene and the larger fragment of the plasmid were ligated with T4 ligase, and the ligated product was transformed into competent cells of the DH5α strain to construct a cloned strain. Inoculate the monoclonal colonies of the cloned strains in LB liquid medium containing 50 μg / ml ampicillin, culture overnight at 37°C with shaking, collect the bacteria for plasmid extraction, PCR identification and double enzyme digestion identification, and transform the correctly identified recombinant vector Into BL21 (DE3) competent cells to construct expression strain 1. The PCR identification map is attached figure 1 .
[0077] Take the transaminase 2 g...
Embodiment 3
[0080] Example 3: Biocatalytic transamination of N-Cbz-3-pyrrolidone to generate (R)-1-benzyloxycarbonyl-3-aminopyrrolidine
[0081] Add 1g of substrate N-Cbz-3-pyrrolidone to a 250ml reaction bottle, add 45ml of phosphate buffer (100mM, pH=8.0), add 10mg of PLP, 4.5g of glucose, 1.45g of D-alanine, 0.1g of coenzyme glucose Hydrogenase, 0.1g coenzyme formate dehydrogenase and 1wt transaminase crude enzyme solution derived from Aspergillus fumigatus (prepared in Example 2), the system pH=8.0, the reaction temperature is 40°C, react with 200rpm for 16h; use 50ml dichloro Methane stopped the reaction, filtered with 50g diatomaceous earth, extracted twice with 50ml dichloromethane, static liquid separation, the organic phase was dried, filtered, and concentrated to obtain the crude product (R)-1-benzyloxycarbonyl-3-aminopyrrolidine: The proportion of system (R)-1-benzyloxycarbonyl-3-aminopyrrolidine is 97-99%, the ee value is greater than 99%, and the yield is 80%-86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com