3:1 type mg/li bimetallic catalyst and its preparation method and application
A technology for bimetallic catalysts and catalysis of aldehydes, applied in the direction of physical/chemical process catalysts, catalytic reactions, chemical instruments and methods, etc., can solve the problems of insufficient catalyst activity, complex catalyst structure, long reaction time, etc., and achieve catalytic reaction time The effect of short, high yield and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
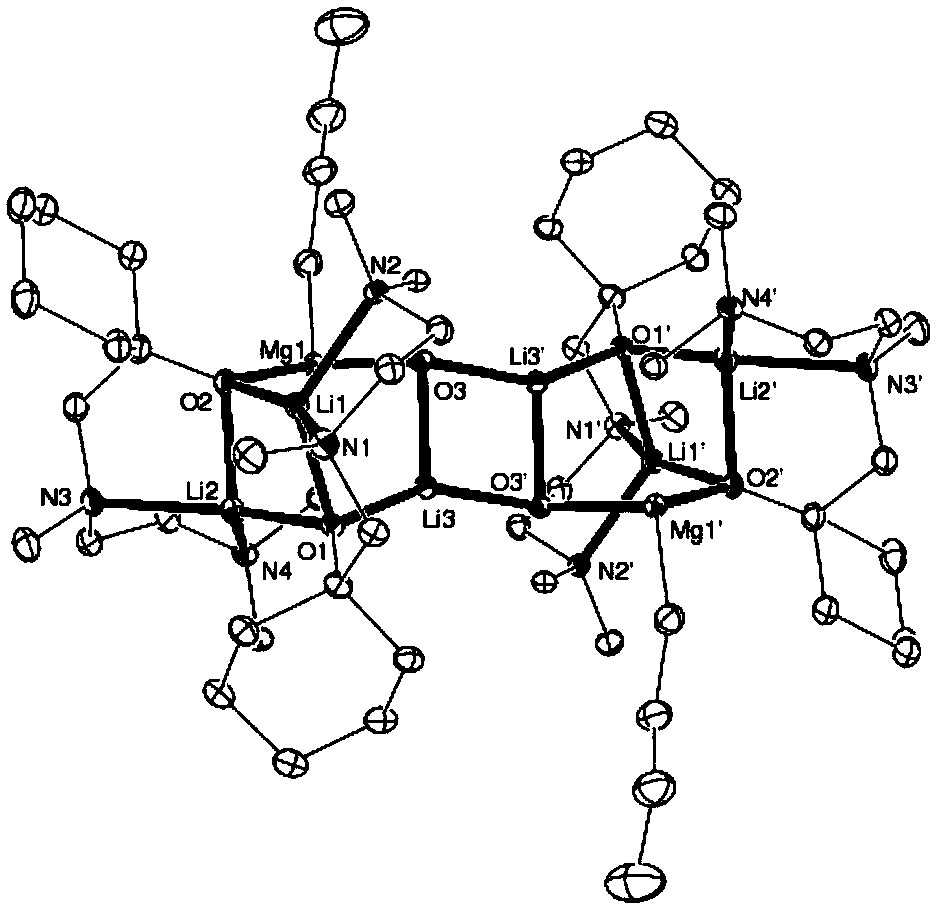
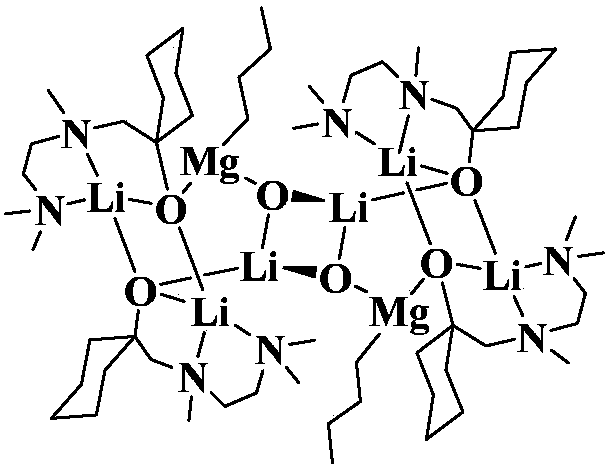
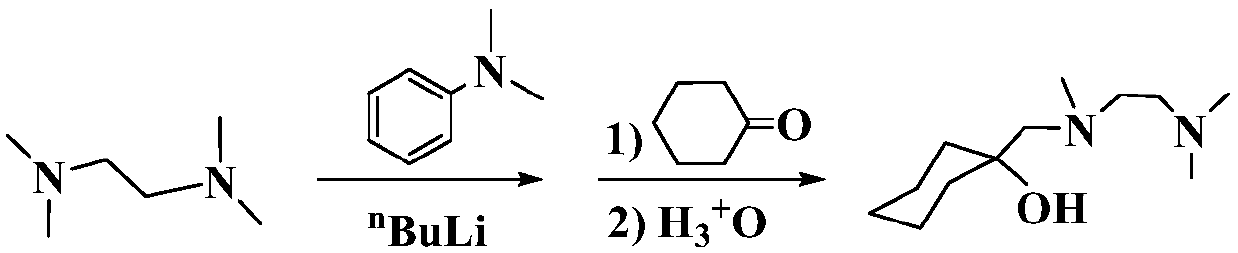
[0016] Example 1 Preparation of 3:1 type N, N, O-tridentate Mg / Li bimetallic catalyst
[0017] Slowly drop n-butyllithium (29.20 mL, 2.5 M, n-hexane solution) into the ether solution of TMEDA (11.02 g, 73 mmol) at 0°C, return to room temperature and react for 2 hours, then add cyclohexanone (7.54 mL, 73mmol) was dropped into the above-mentioned reaction solution to continue the reaction for 2 hours, then hydrolyzed under acidic conditions, and the aqueous phase obtained after liquid separation was neutralized to weak alkalinity with 5% aqueous sodium hydroxide solution, then extracted three times with anhydrous ether, organic The phase was dried with anhydrous magnesium sulfate and distilled under reduced pressure, and the colorless fraction at 78°C was collected to obtain the N,N,O-tridentate aminoalcohol ligand with a yield of 84%;
[0018] Under the protection of an inert gas, the N,N,O-tridentate aminoalcohol ligand (2.0mmol, 0.43g) was dissolved in 10mL of ether solvent, ...
Embodiment 2
[0021] (1) the preparation of catalyst is the same as embodiment 1
[0022] Catalytic reduction of benzaldehyde to benzyl alcohol (MPV): under nitrogen protection at room temperature, benzaldehyde (0.42g, 4.0mmol) and isopropanol (0.37mL, 4.8mmol) were added to a nitrogen-protected 3:1 type N,N, In the O-tridentate Mg / Li bimetallic catalyst (1.06g, 0.2mmol), after reflux stirring reaction for 1 hour, then terminate the reaction with water, extract with ether, dry, after rotary evaporation, separate with column chromatography (mobile phase 7:3 mixed solvent of n-hexane and ethyl acetate), benzyl alcohol after concentration. Yield 94%. 1 HNMR (300MHz, CDCl 3 ): δ(ppm)3.62(s,1H,OH),4.55(s,2H,CH 2 ),6.86-7.39(m,5H,C 6 h 5 ).
Embodiment 3
[0024] (1) the preparation of catalyst is the same as embodiment 1
[0025] (2) Catalytic reduction of acetophenone to 1-phenylethanol (MPV): under nitrogen protection at room temperature, acetophenone (0.48g, 4.0mmol) and isopropanol (0.37mL, 4.8mmol) were added to nitrogen-protected 3 : Type 1 N, N, O-tridentate Mg / Li bimetallic catalyst (1.06g, 0.2mmol), reflux and stir the reaction for 1 hour, then stop the reaction with water, extract with ether, dry, rotary evaporate, and use column Separation by chromatography (the mobile phase is a 7:3 mixed solvent of n-hexane and ethyl acetate), and concentrated to 1-phenylethanol with a yield of 91%. 1 H NMR (300MHz, CDCl 3 ): δ(ppm)1.48(d,3H,CH3),3.62(s,1H,OH),4.65(s,H,CH),7.13-7.45(m,5H,C 6 h 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com