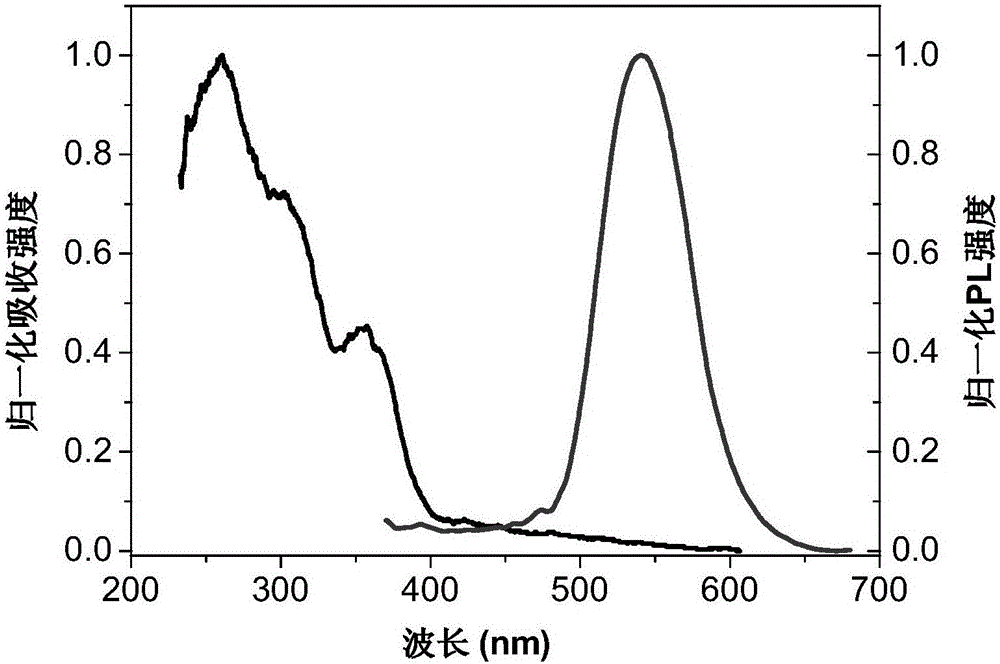
2-(2'-hydroxyphenyl) benzoxazole compound containing substituent at 4-site and preparation method and application thereof
A technology of hydroxyphenyl and benzoxazole, applied in the field of organic semiconductor optoelectronics, can solve the problems of high production cost, complex preparation process, poor repeatability, etc., and achieve the effects of large Stokes shift, simple synthesis and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] First, the preparation of 2-(2'-4-bromophenol)benzothiazole:
[0038] Get diaminophenol (4.4g, 20mmol, 1equiv) and p-aminobenzoic acid (2.5g, 20mmol, 1equiv) and add in 130ml polyphosphoric acid, in N 2 Heated to 168°C in an ambient environment, and refluxed for 6 hours. After the reaction was complete, the product was dissolved in 100 ml of ice-water mixture after cooling, and saturated NaOH aqueous solution was added thereto for neutralization. When the pH value is adjusted to about 6, add NaHCO 3 , while adding and stirring until there is no more CO 2 Bubbles are generated. The neutralized solution was left to stand until the solution was clarified and then suction filtered. Add the filtered solid to saturated NaHCO 3 The aqueous solution was extracted three times with ethyl acetate, the combined organic layers were washed with anhydrous Na 2 SO 4 After drying and rotary evaporation, 5 g of green product was obtained with a yield of 83%. (In the following exa...
Embodiment 2
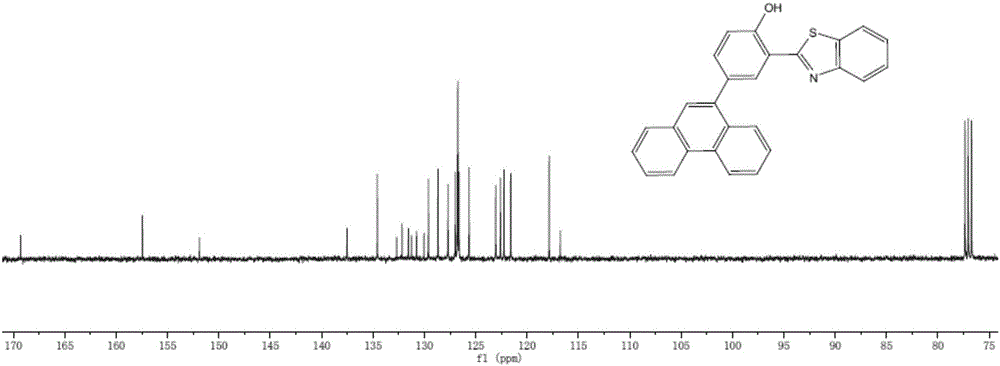
[0043]Preparation of 2-(2'-benzothiazole)-4-(9'-phenanthrene)phenol
[0044] (1) Borylation of compounds containing aromatic hydrocarbon conjugated structures: under the protection of nitrogen, 9-bromophenanthrene was dissolved in dry tetrahydrofuran, placed in a dry ice acetone bath and cooled to -78°C, and n-butyllithium was added at low temperature, 9 -The addition ratio of phenanthrene bromide and n-butyllithium is 1:1.2. After stirring for 3 hours, add n-butyl borate, borate, continue the reaction for 0.5 hours, remove the dry ice acetone bath, heat up to room temperature and react for 18 hours. After the reaction was completed, the reaction was quenched in an ice-water bath, and then the organic phase was extracted with water and dichloromethane, and the organic phases were combined, dried and spin-dried to obtain the product.
[0045] (2) Take 9-phenanthrene borate (0.18g, 0.75mmol, 1 equivalent) and 2-(2'-4-bromophenol) benzothiazole (0.24g, 0.75mmol, 1 equivalent) and...
Embodiment 3
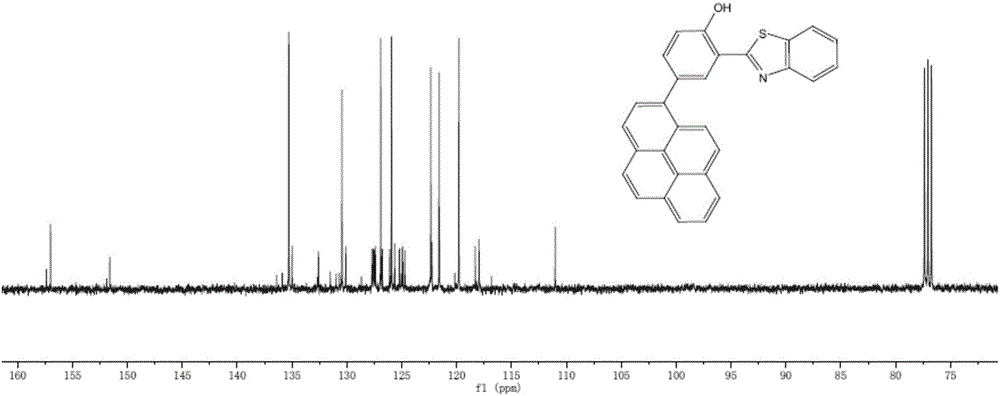
[0048] Preparation of 2-(2'-benzothiazole)-4-(1'-pyrene)phenol
[0049] 9-phenanthrene borate in embodiment 2 is changed into 1-pyrene borate, and concrete steps are:
[0050] (1) Borylation of compounds containing aromatic hydrocarbon conjugated structures: under the protection of nitrogen, 1-bromopyrene was dissolved in dry tetrahydrofuran, placed in a dry ice acetone bath and cooled to -78 ° C, and n-butyllithium was added at low temperature, 1 - The addition ratio of bromopyrene and n-butyllithium is 1:1.2. After stirring for 3 hours, add n-butyl borate, borate, continue the reaction for 0.5 hours, remove the dry ice acetone bath, heat up to room temperature and react for 18 hours. After the reaction was completed, the reaction was quenched in an ice-water bath, and then the organic phase was extracted with water and dichloromethane, and the organic phases were combined, dried and spin-dried to obtain the product.
[0051] (2) Take 9-pyrene borate (0.6g, 2.2mol, 1 equival...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com