Total chemical synthesis method of adenine
A technology for chemical synthesis and adenine, applied in the field of chemical synthesis of adenine, can solve the problems of high raw material price, long synthesis route, low total yield, etc., achieve low equipment requirements, reduce three waste pollution and cost, and simple and easy raw materials the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
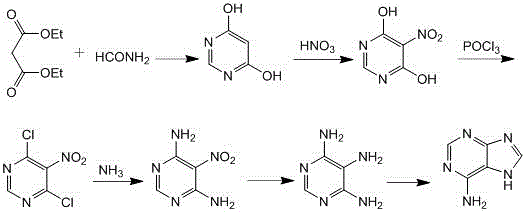
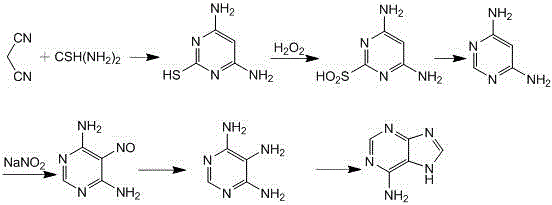
Image
Examples
Embodiment 1
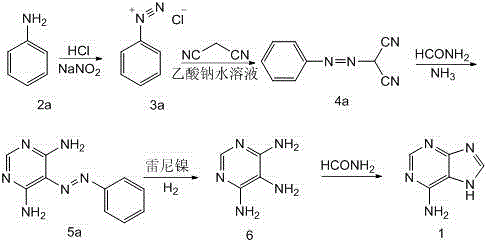
[0044] The reaction formula is as follows:
[0045]
[0046] 1) Synthesis of 2-phenylazomalononitrile (Formula 4a)
[0047] Add 250g of concentrated hydrochloric acid and 600mL of water into a 2000mL four-necked reaction flask, cool to below 10°C, add 96g of aniline (Formula 2a) dropwise, continue cooling to 0°C, control the temperature at 0-5°C, and add 73.5g of sodium nitrite dropwise (dissolved in 180mL of water) solution, drop it for about 1 hour, and keep it at 5°C for 1 hour to form a diazonium salt (Formula 3a) solution. Add 60 g of malononitrile, stir for half an hour to dissolve and clarify, add dropwise a saturated aqueous solution of sodium acetate, adjust the pH value of the solution, keep warm for 2 hours, filter, rinse the filter cake with water, and dry to obtain 142.7 g of light yellow solid after recrystallization. -Benzodiazomalononitrile (formula 4a), yield 92.3% (according to the amount of malononitrile), m.p.134~136°C.
[0048] 2) Synthesis of 3,5-dia...
Embodiment 2
[0056] The reaction formula is as follows:
[0057]
[0058] 1) Synthesis of 2-(p-tolyl)azomalononitrile (Formula 4b)
[0059] Add 250g of concentrated hydrochloric acid and 600mL of water into a 2000mL four-necked reaction flask, cool to below 10°C, add 110.5g of p-methylaniline (Formula 2b) dropwise, continue to cool to 0°C, control the temperature at 0-5°C, and dropwise add Sodium nitrate solution of 73.5g (dissolved in 180mL water) was dripped in about 1 hour, and then kept at 5°C for 1 hour to form a diazonium salt (Formula 3b) solution. Add malononitrile 60g, after stirring for half an hour to dissolve and clarify, add dropwise a saturated aqueous solution of sodium acetate, adjust the pH value of the solution, keep warm for 2 hours, filter, rinse the filter cake with water, dry to obtain 155.8g light yellow solid after recrystallization ( Formula 4b), the yield is 93.1% (based on the amount of malononitrile), m.p.170-172°C.
[0060] 2) Synthesis of 3,5-diamino-4-(p...
Embodiment 3
[0068] The sodium acetate saturated aqueous solution in the example one 1) is replaced by the potassium phosphate saturated aqueous solution, and other feeding ratios and operating methods are unchanged, and 147g of light yellow solid 2-benzazinomalononitrile is obtained, and the yield is 95.1% (according to propane Dinitrile meter).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com