Peptide for repairing cartilage and/or treating osteoarthritis
A technology for osteoarthritis and cartilage, applied in the field of peptides and drugs containing the peptides, can solve the problems of difficulty in inducing activity, limited clinical application of BMP-2, difficulty in maintaining therapeutic concentration, etc., and achieve improved efficacy in vivo Duration, promotion of chondrocyte proliferation, and stability improvement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
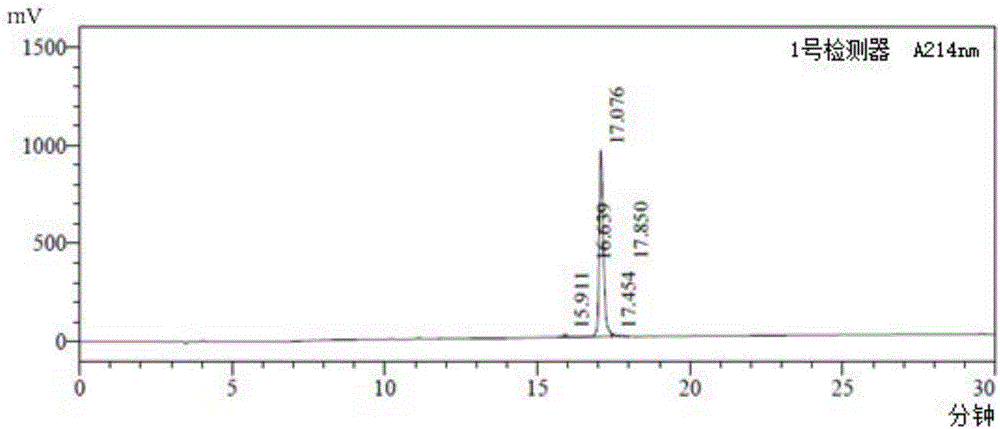
[0037] The solid-phase synthesis of embodiment 1 BM23 peptide
[0038] BM23 peptide (SEQ ID NO: 1, in which amino acids 1, 7 and 23 use D-glutamine, homoarginine and amidated leucine respectively) commissioned by Shanghai Aobo Biotechnology Co., Ltd. The company adopts conventional solid-phase process synthesis, and the purity of the synthesized peptide is >98%, see figure 1 .
Embodiment 2
[0039] Example 2 Isolation and cultivation of mesenchymal stem cells (BMSCs)
[0040] Three 8-week-old SD rats were killed by dislocation and sterilized with 70% ethanol for 5 minutes. The layers of the abdominal wall are then cut with scissors, the incision is drawn to the femur, and the muscles on the femur are carefully separated.
[0041] Cut off the middle of the femur, draw low-sugar DMEM culture solution (containing 1 mL / L heparin) with a syringe and repeatedly flush the bone marrow cavity to flush out the bone marrow.
[0042] The bone marrow fluid was flushed repeatedly with a syringe, passed through No. 7 and No. 4 needles in turn to make a single-cell suspension.
[0043] The single cell suspension was centrifuged (1500r / min, 10 minutes), and the supernatant was discarded. Add DMEM-F12 complete medium containing 10% FBS to 10 7 The density of each / mL was inoculated in a culture flask, placed at 37°C, 5% CO 2cultured in an incubator.
[0044] Cells were purified...
Embodiment 3
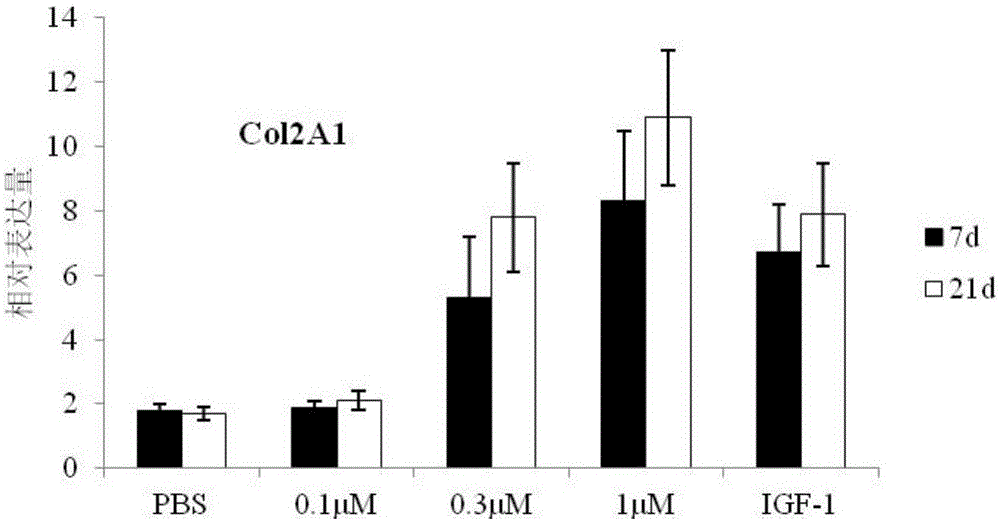
[0048] Example 3 The effect of BM23 peptide on the differentiation of mesenchymal stem cells in vitro
[0049] chondrogenic induction
[0050] The BM23 peptide was dissolved in phosphate buffered saline (PBS).
[0051] The cells were resuspended in complete chondrogenic medium (purchased from Guangzhou Saiye Biotechnology Co., Ltd., product number: RASMX-90011) containing different concentrations of BM23 peptide (0.1, 0.3 and 1 μM), so that the concentration of BMSCs was 5.0× per ml. 10 5 cells. In addition, PBS was used as a negative control, and 1 μM human insulin growth factor (IGF-1, purchased from Beijing Sino Biological Technology Co., Ltd.) was used as a positive control.
[0052] The treated BMSCs were incubated at 37°C and 5% CO2 saturated humidity. Change the medium every 2-3 days and add 0.5 mL of fresh complete chondrogenic medium to each tube.
[0053] Generally, the samples can be collected after 28 days of continuous induction, and the cartilage balls can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com