Preparation method for guided tissue regeneration membrane for periodontitis repair
A technology for guiding tissue regeneration and periodontitis, applied in the field of preparation of guiding tissue regeneration membrane, can solve the problems of restricting the use of regeneration membrane, unfavorable bone repair, matching the degradation speed of bone tissue repair speed, etc. Responsiveness, enhanced adhesion and proliferation, improved biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
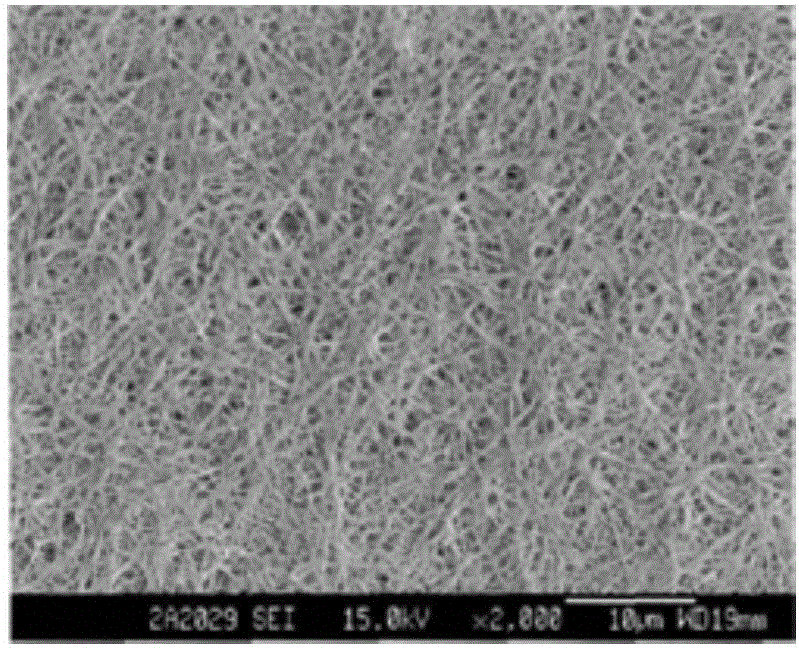
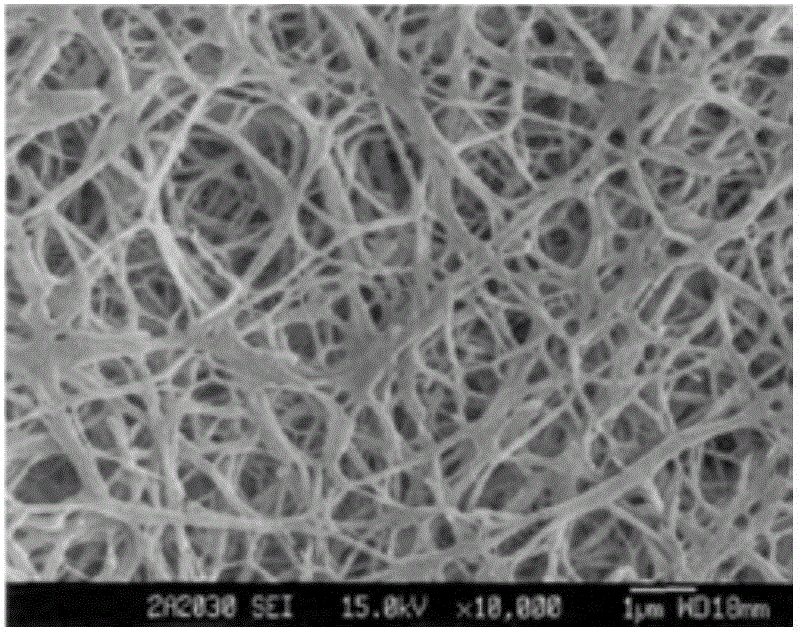
[0026] Embodiment 1: Composite membrane preparation
[0027] (1) Add 4.17g serine, 1250g lactic acid and 1g catalyst stannous isooctanoate in the reaction vessel, react at 150°C under vacuum and reduced pressure until no water is distilled out, and the prepared molecular weight is 1955, and the intrinsic viscosity is 0.138 Low molecular weight lactic acid-serine polymer (PLAS), the synthetic PLAS is 4000~500cm -1 Infrared spectroscopy scans in the wavenumber range. In the infrared spectrum, 3340cm -1 The sharp absorption peak at represents the stretching vibration of the amino group in the polymer; 2943cm -1 The absorption peak at indicates that there is a saturated C-H stretching vibration in the polymer, and at 1361cm -1 There is an absorption peak at , indicating the presence of methyl-CH in the polymer 3 ;1746cm -1 The absorption peak at represents the existence of functional group C=O; 1266cm -1 The absorption peaks indicated the presence of C-O structure; these abs...
Embodiment 2
[0030] Embodiment 2: the biological barrier effect test of composite film
[0031] Use a punching machine to cut each group of membrane materials into small discs with a diameter of 6mm, all are fine 60 Co irradiated for 24 hours to sterilize, and irradiated with ultraviolet light for 30 minutes before use. Put the material into a 96-well culture plate 1 day before the experiment, pre-wet it with the culture medium, and set it aside.
[0032] Take the human gingival fibroblasts cultured until the cell density reaches about 80% of the culture bottle, digest with trypsin, count, and adjust the concentration of the cell suspension to 5×l0 3 / ml. Take 100 μL and inoculate it on the pre-wetted membrane material in the 96-well plate of each group, mark the side of the membrane material inoculated with cells as the front side, and the other side as the reverse side. 2 1. Cultivate under saturated humidity conditions, add 100 μL of culture solution to each well after 3 hours, conti...
Embodiment 3
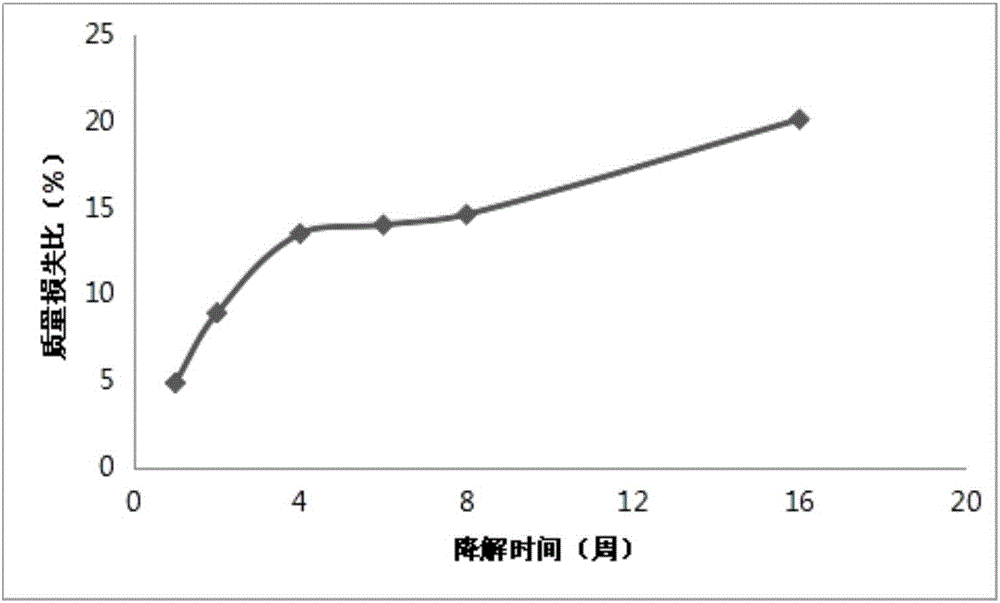
[0035] Embodiment 3: In vitro degradation test of composite membrane
[0036] Through the in vitro degradation experiment of the composite film, its biodegradation behavior was studied, and whether it could meet the requirement that the oral barrier film needs to exist in the body for 4-6 months.
[0037] The experimental steps of the degradation test are as follows: ① prepare phosphate buffered saline; ② vacuum desiccate the sample to constant weight. The sample is cut into 2*5mm strips, divided into 20 parts, each with a mass of about 0.3000g, and recorded W 0 ;③ Add 15ml of buffer solution to 20 15ml screw-top bottles and number them, and put the samples in order; , 8, and 16 weeks to take out three samples; ⑤ vacuum-dry the filter paper to constant weight and record Wc, each sample is filtered by quantitative filter paper; ⑥ measure and record the mass Wd of the clean and dry centrifuge tube; ⑦ add the filtrate to the centrifuge tube After centrifuging for 10 minutes, ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com