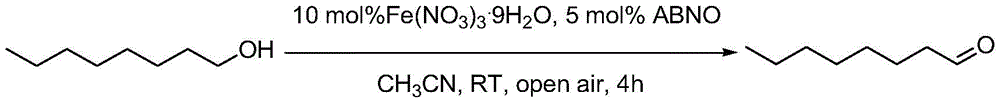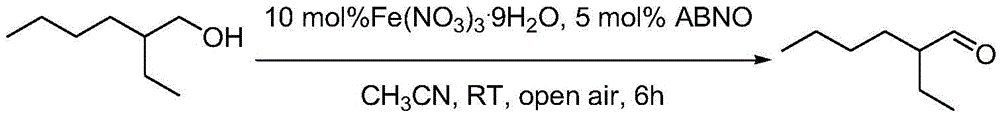Green method for preparation of aldehyde or ketone by iron catalyzed alcohol oxidation
An iron-catalyzed alcohol, green technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of limited application potential, impact on application, low activity of aliphatic primary alcohols, etc. Easy-to-operate, easy-to-react effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0025] Example 1 Oxidation of 1-octanol to 1-octanal
[0026]
[0027] Take successively Fe(NO3)3.9H2O (40.4mg, 10mol%), ABNO (7mg, 5mol%), 1-octanol (130mg, 1mmol) and join in the reaction test tube of 10ml, then add 2ml acetonitrile as solvent, room temperature Lower the open reaction, and then use GC-MS to detect the degree of reaction. After the reaction was completed, internal standard biphenyl was added, and the yield of product 1-octanal was quantitatively analyzed by GC. After 4 hours of reaction, the yield of 1-octanal was 90%, and the selectivity was greater than 99%.
Embodiment 2
[0028] Example 2 Oxidation of 2-ethylhexanol to 2-ethylhexanal
[0029]
[0030] Fe(NO3)3.9H2O (40.4mg, 10mol%), ABNO (7mg, 5mol%), 2-ethylhexanol (130mg, 1mmol) were successively added to a 10ml reaction test tube, and then 2ml of acetonitrile was added as a solvent , open the reaction at room temperature, and then use GC-MS to detect the degree of reaction. After the reaction was completed, the internal standard biphenyl was added, and the yield of the product 2-ethylhexanal was quantitatively analyzed by GC. After 6 hours of reaction, the yield of 1-octanal was 86%, and the selectivity was 95%.
Embodiment 3
[0031] Example 3 Oxidation of 1-hexanol to 1-hexanal
[0032]
[0033] Fe(NO3)3.9H2O (40.4mg, 10mol%), ABNO (7mg, 5mol%), 1-hexanol (102mg, 1mmol) were successively added to a 10ml reaction test tube, then 2ml of acetonitrile was added as a solvent, and the Lower the open reaction, and then use GC-MS to detect the degree of reaction. After the reaction was finished, the internal standard biphenyl was added, and the yield of the product 1-hexanal was quantitatively analyzed by GC. After 6 hours of reaction, the yield of 1-hexanal was 95%, and the selectivity was greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com