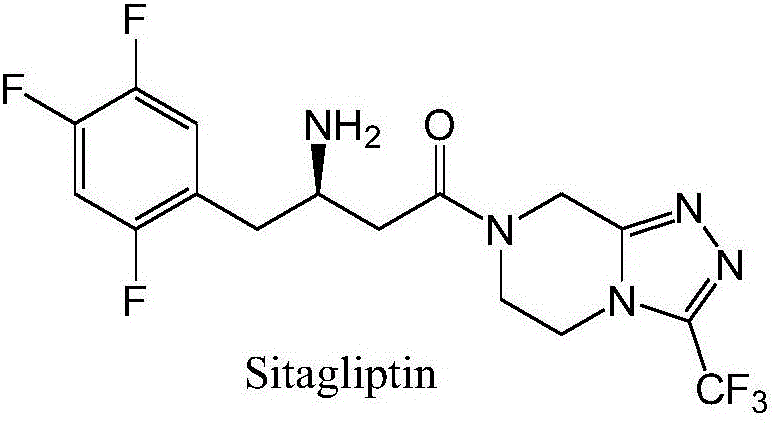
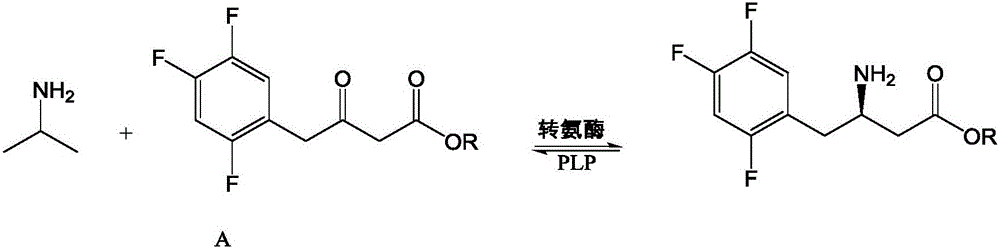
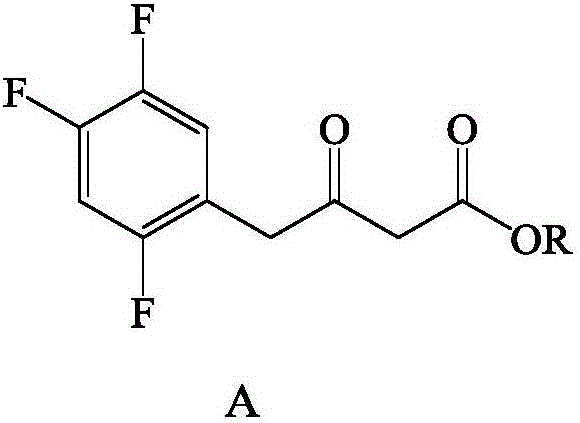
Recombinant transaminase as well as preparation method and application of recombinant transaminase
A transaminase and amino acid technology, applied in the field of bioengineering, can solve problems such as low product yield and chiral purity, harsh reaction conditions, and long synthetic routes, and achieve excellent stereoselectivity, high reaction conversion rate, and high product yield. The effect of mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The establishment of embodiment 1 genetically engineered bacteria
[0031] According to the gene sequence of Aspergillus terreus (Aspergillus terreus NIH2624) transaminase recorded in Genbank (NCBI accession number: XM_001209325.1), the gene fragment was artificially synthesized, and the fragment was amplified by PCR (adding NdeI and BamHI endonuclease genes on both sides of the fragment) Fragment), its nucleotide sequence is shown in SEQ ID NO.3. And the gene was inserted into the pET21a plasmid by using the NdeI and BamHI endonuclease sites, and the ligated vector was transferred into Escherichia coli BL21 (DE3) to establish transaminase genetically engineered bacteria. The primers for PCR amplification of the transaminase gene are: forward primer F1: GGGGCCATATGGCCTCCATGGACAAAGTCTTT (SEQ ID NO.4), reverse primer R1: GGGCCGGATCCCGTTATAATCAATCTCGAAGC (SEQ ID NO.5).
Embodiment 2
[0032] The acquisition of embodiment 2 transaminase mutant gene
[0033] In this study, protein engineering of transaminases was carried out using error-prone PCR method. Error-prone PCR is to adjust the reaction conditions when using DNA polymerase to amplify the target gene, such as increasing the concentration of magnesium ions, adding manganese ions, changing the concentration of four kinds of dNTPs in the system, or using low-fidelity DNA polymerase, etc. To change the mutation frequency in the amplification process, so as to randomly introduce mutations into the target gene at a certain frequency, and obtain random mutants of protein molecules. In this study, the principle that a polymerase with low fidelity can easily incorporate random mutations into the amplified product under specific measures was adopted, and at the same time, Mn 2+ Alternative to natural cofactor Mg 2+ increase the probability of error.
[0034] The 50 μl PCR reaction system is: 5 μl of 10× ampl...
Embodiment 3
[0042] The shake flask preparation of embodiment 3 transaminase
[0043] The recombinant Escherichia coli constructed in Example 1 and Example 2 were respectively inoculated into 50 mL of LB medium containing ampicillin (100 μg / mL) (peptone 10 g / L, yeast extract 5 g / L, NaCl 10 g / L, pH 7.2 ) in a shaker at 37°C and 200 rpm for more than 16 hours. Transfer 2 mL of bacterial culture solution to 50 mL of LB medium containing chloramphenicol (or ampicillin), place it under the same conditions for shaking culture, and regularly measure the absorbance value of the bacterial liquid at 600 nm to monitor the growth density of the bacterial cells. When the OD 600 value of the bacterial solution was 0.6-0.8, the inducer IPTG was added to a final concentration of 0.2 mmol / L, and the expression was induced at 30°C for more than 12 hours. After expression, the cells were collected by centrifugation (5000rpm, 15min, 4°C), washed twice with phosphate buffer (pH7.2, 50mM), dispersed in the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com