Preparation method of alkaline manganese chloride
A manganese chloride, basic technology, applied in the field of animal feed additives, can solve the problems of unstable chemical properties, low bioavailability, not easy to absorb, etc., and achieve the effects of low production cost, good fluidity and comprehensive functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
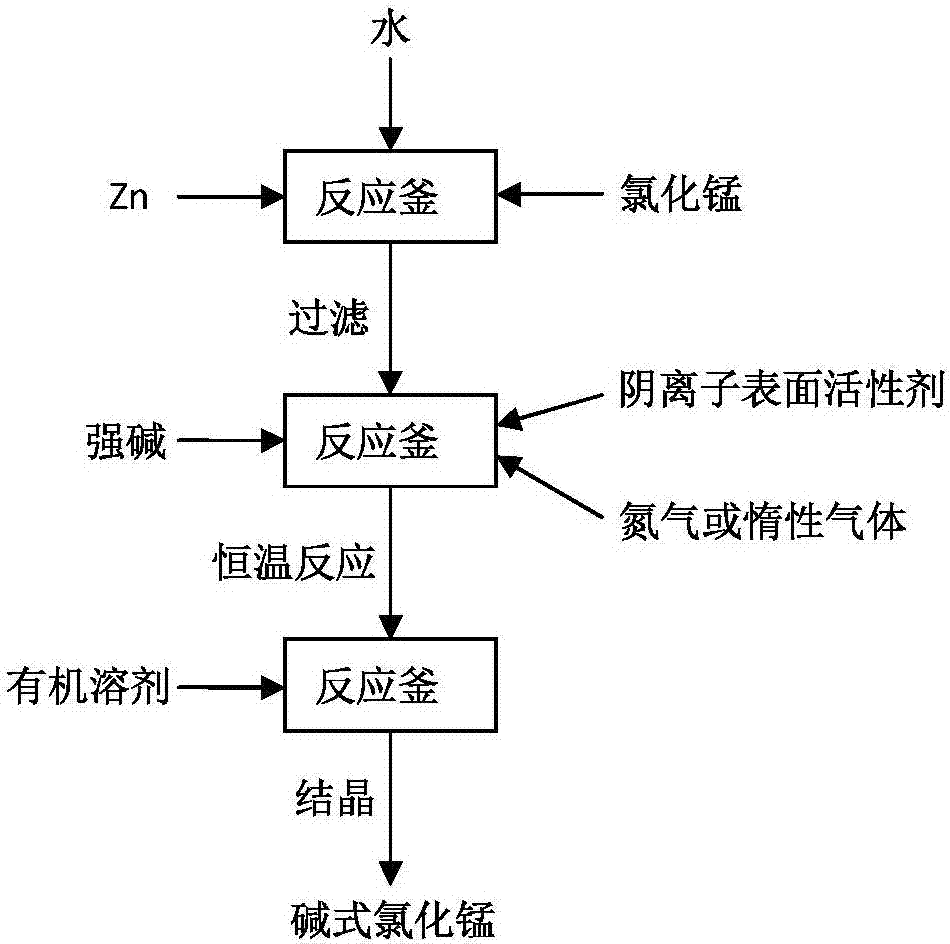
[0041] A kind of preparation method of basic manganese chloride, such as figure 1 shown, including the following steps:
[0042] (1) 262.5Kg manganese chloride with a purity of 96% is added to a 2m 3 Completely dissolve in the ceramic reactor, adjust the pH value of the solution to 7.5, add 288g of manganese powder, stir for 1 hour and filter, repeat the above operation once;
[0043] (2) Place the filtrate filtered in (1) in the ceramic reactor again, and add 2Kg sodium lauryl sulfate in the reactor, fill it with nitrogen protection, slowly add the concentration of 20% under constant stirring , 404Kg of sodium hydroxide solution with a purity of 99%, after all the sodium hydroxide solution is added, the temperature is raised to 70°C and then reacted at a constant temperature for 10 hours;
[0044] (3) After the reaction finishes, add 13.3Kg alcohol to the closed reactor, and cool to room temperature crystallization under stirring;
[0045] (4) After press filtration, wash ...
Embodiment 2
[0051] A preparation method for basic manganese chloride, comprising the following steps:
[0052] (1) 262.5Kg manganese chloride with a purity of 96% is added to a 2m 487.5Kg water 3 Completely dissolve in the ceramic reaction kettle, adjust the pH value of the solution to 7.0, add 97.3g of manganese powder, stir for 0.2h and then filter, repeat the above operation once;
[0053] (2) the filtrate after filtering in (1) is placed in the ceramic reactor again, and the 320g fatty alcohol sodium sulfate that adds in the reactor, after charging into nitrogen protection, slowly adding concentration under constant stirring is 50%, Potassium hydroxide solution with a purity of 99% was 188.5Kg. After all the potassium hydroxide solution was added, the temperature was raised to 30°C and then reacted at a constant temperature for 24 hours;
[0054] (3) After the reaction finishes, add 58Kg glycerol to the closed reactor, and cool to room temperature crystallization under stirring;
[...
Embodiment 3
[0061] A preparation method for basic manganese chloride, comprising the following steps:
[0062] (1) 646.5Kg manganese chloride tetrahydrate with a purity of 98% is added to a 5m tank with 2.33t of water 3 Completely dissolve in the ceramic reactor, adjust the pH value of the solution to 6.0, add 142g of manganese powder, stir for 0.5h and then filter, repeat the above operation once;
[0063] (2) the filtrate after filtering in (1) is placed in the ceramic reaction kettle again, and the 1.5Kg fatty alcohol ether sodium sulfate that adds in the reaction kettle, after filling into argon protection, slowly add concentration under constant stirring. 30%, 235.1Kg of lithium hydroxide solution with a purity of 99%, after all the lithium hydroxide solution is added, the temperature is raised to 50°C and then reacted at a constant temperature for 4 hours;
[0064] (3) After the reaction finishes, add 140Kg ethyl acetate to the closed reaction kettle, be cooled to room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com