Separation method of polyol mixture
A separation method and mixture technology, which are applied in the preparation of organic compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., to achieve the effects of reducing vacuum degree, reducing high performance requirements, reducing the number of trays and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
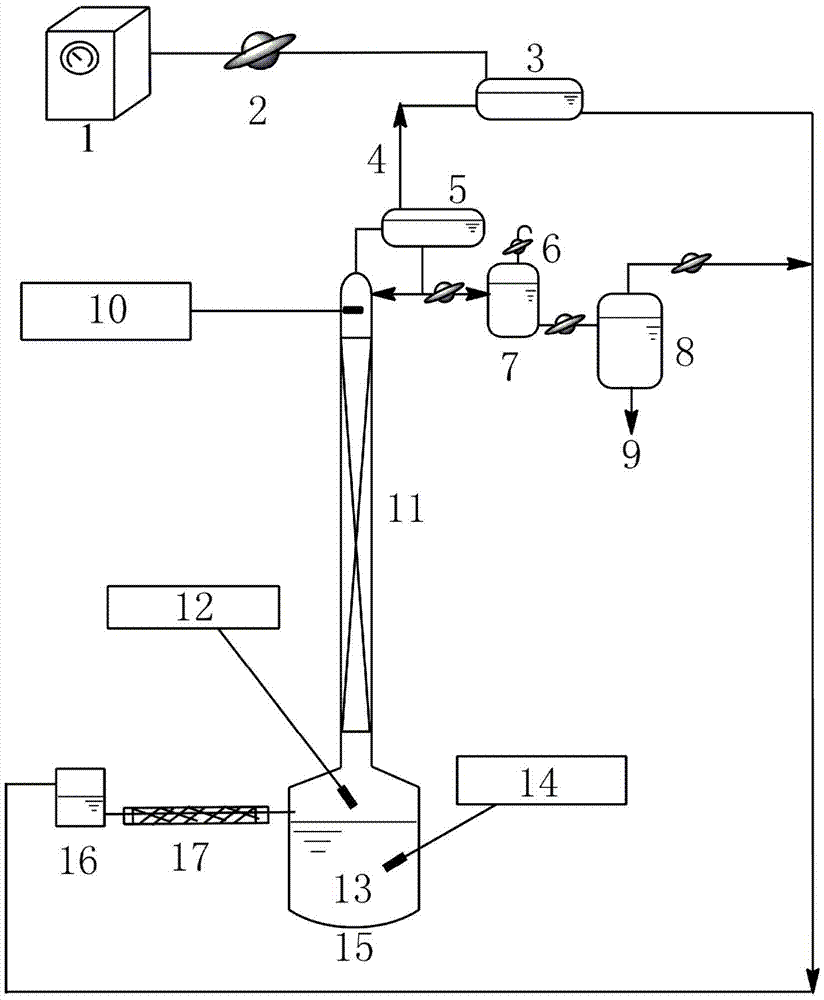
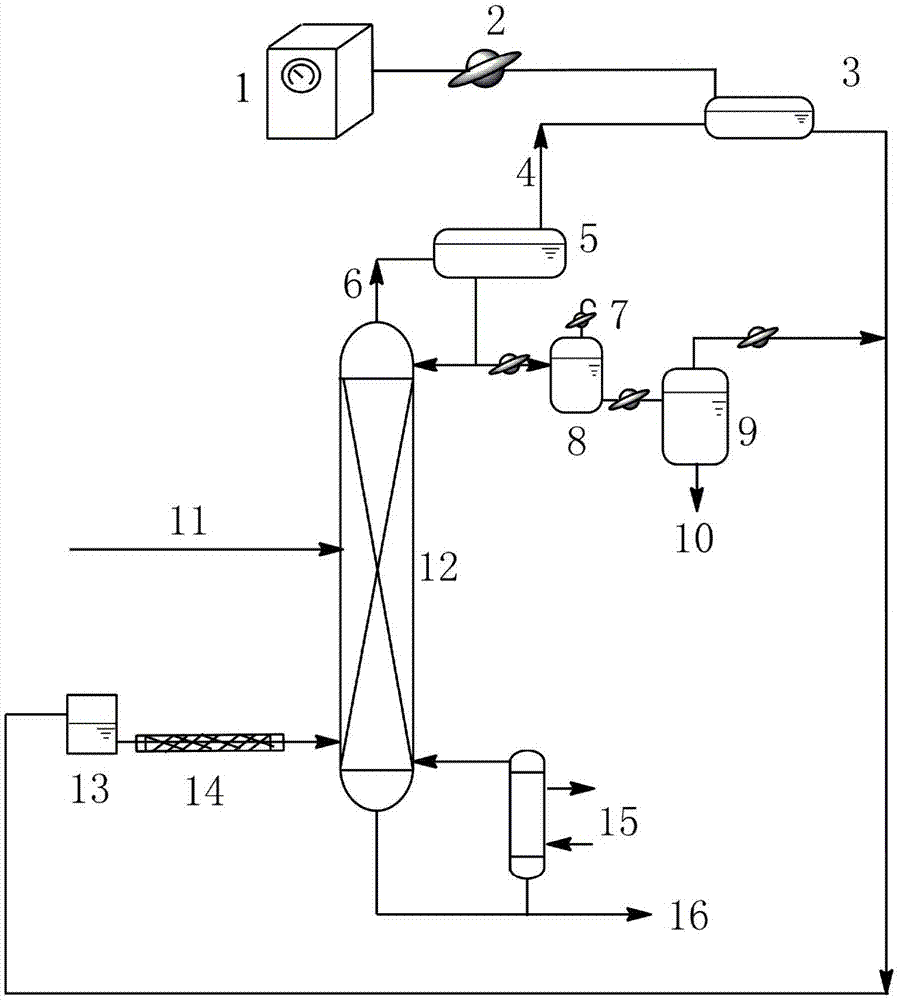
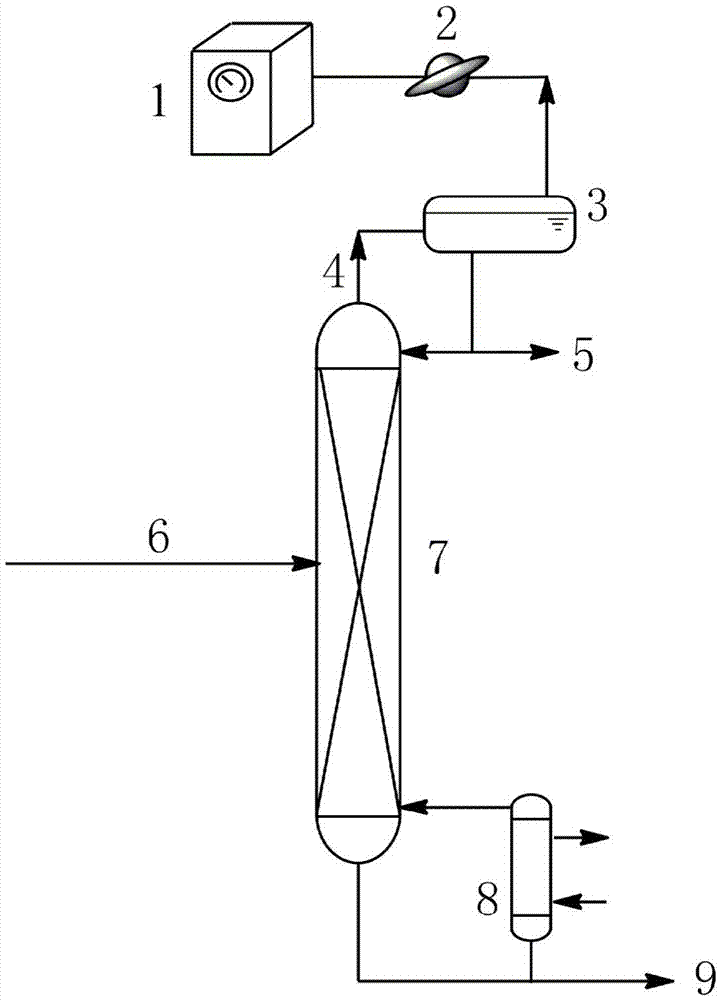
[0029] Such as figure 1 As shown, add 200mL of a mixture of 1,2-propanediol and ethylene glycol to the bottom of the batch distillation column, wherein the mass fraction of 1,2-propanediol is 22%, and the mass fraction of ethylene glycol is 78%. The absolute pressure of the gas phase at the top of the rectification tower is maintained at 13kPa, and the liquid phase temperature in the tower kettle is 120°C. The rectification column uses triangular spiral packing, and the number of theoretical plates is 55. Adjust the temperature of condenser 1 to 100°C and the temperature of condenser 2 to 10°C. Total reflux for 0.5h to stabilize the system;
[0030]Set the reflux ratio to 10:1. Inject ethyl butyrate from the storage tank at a flow rate of 0.78 mL / min into the evaporation tube with an inner surface temperature of 105 °C, and the ethyl butyrate is completely vaporized in the evaporation tube. The gaseous extractant enters the distillation column where it contacts the descend...
Embodiment 2
[0032] Such as figure 1 As shown, add 200mL of a mixture of 1,2-butanediol and ethylene glycol to the bottom of the batch distillation column, wherein the mass fraction of 1,2-butanediol is 10%, and the mass fraction of ethylene glycol is 90%. The absolute pressure of the gas phase at the top of the rectification tower is kept at 14.5kPa, and the temperature of the liquid phase in the tower kettle is 110°C. The rectification column uses Pall ring packing, and the number of theoretical plates is 60. Adjust the temperature of condenser 1 to 90°C and the temperature of condenser 2 to 3°C. Full reflux for 0.5h to stabilize the system;
[0033] Set the reflux ratio to 10:1. Inject propyl ether from the storage tank at a flow rate of 1.1 mL / min into the evaporating tube with an inner surface temperature of 95°C. Most of the propyl ether is vaporized in the evaporating tube, and a small amount is vaporized at the bottom of the tower. The gaseous extractant enters the distillatio...
Embodiment 3
[0035] Such as figure 1 As shown, add 200mL of a mixture of 1,2-propanediol and ethylene glycol to the bottom of the batch distillation column, wherein the mass fraction of 1,2-propanediol is 50%, and the mass fraction of ethylene glycol is 50%. The absolute pressure of the gas phase at the top of the rectification tower is maintained at 19.8kPa, and the temperature of the liquid phase in the tower kettle is 150°C. The rectification column uses Raschig ring packing, and the number of theoretical plates is 83. Adjust the temperature of condenser 1 to 137°C and the temperature of condenser 2 to 20°C. Total reflux for 0.7h to stabilize the system;
[0036] Set the reflux ratio to 8.5:1. From the storage tank, inject isobutanol into the evaporation tube with an inner surface temperature of 143 °C at a flow rate of 1 mL / min. Most of the isobutanol is vaporized in the evaporation tube, and a small amount is vaporized at the bottom of the tower. The gaseous extractant enters the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com