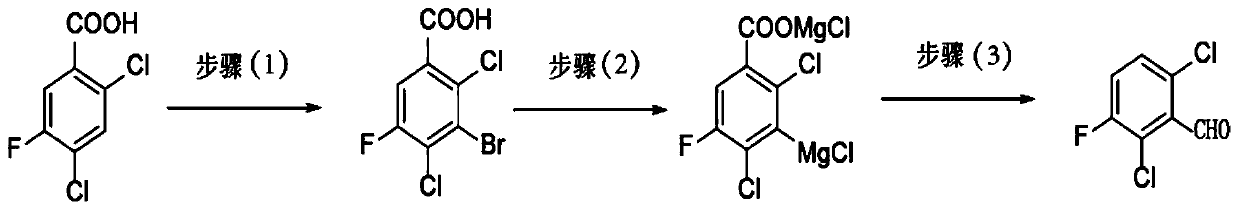
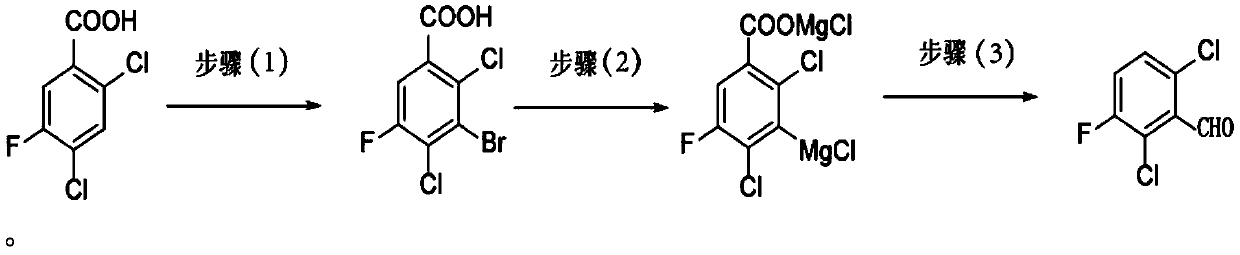
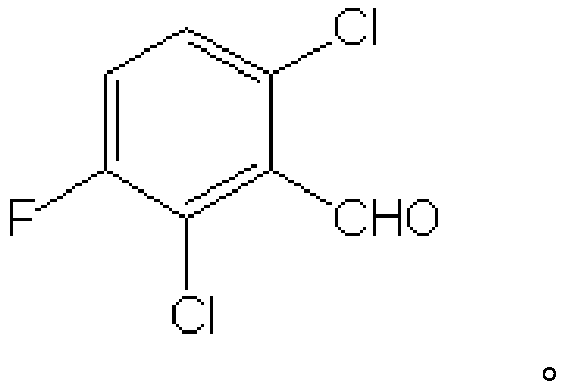
Preparation method of 2,6-dichloro-3-fluorobenzaldehyde and preparation method of fluoroquinolones
A technology of fluorobenzaldehyde and fluorobenzoic acid, which is applied in the preparation of organic compounds, carbon-based compounds, magnesium organic compounds, etc., can solve the problems of complicated operation, difficult to obtain raw materials, expensive raw materials, etc., and achieve simple preparation process, Good economic value and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the present invention uses 2,4-dichloro-5-fluorobenzoic acid as a raw material, reacts in a concentrated sulfuric acid solvent under the action of a brominating reagent to generate 2,4-dichloro-3-bromo-5- Fluorobenzoic acid; Grignard for the preparation of 2,4-dichloro-3-bromo-5-fluorobenzoic acid by halogen-metal exchange reaction between 2,4-dichloro-3-bromo-5-fluorobenzoic acid and Grignard reagent Reagent, and further formylation reaction, then decarboxylation to obtain the target compound 2,6-dichloro-3-fluorobenzaldehyde, specifically, the steps of the preparation method of the present invention are as follows:
[0034] (1) 2,4-dichloro-5-fluorobenzoic acid and brominated reagent are dissolved in concentrated sulfuric acid and reacted to obtain 2,4-dichloro-3-bromo-5-fluorobenzoic acid;
[0035] In a preferred embodiment, the brominated reagent used is N-bromosuccinimide (NBS).
[0036] In a preferred embodiment, the molar ratio of NBS t...
Embodiment 1
[0060] Prepare 2,6-dichloro-3-fluorobenzaldehyde according to the method described below, and the specific steps are as follows:
[0061] (1) Add 96g of concentrated sulfuric acid with a mass concentration of 98% and 19.2g of 2,4 dichloro-5-fluorobenzoic acid into the reaction flask, and heat to 30-35°C, stir to dissolve;
[0062] Then, add 19.7gNBS in batches, after adding, keep warm for 12 hours, TLC (ethanol / ethyl acetate=1 / 1) analysis, raw material reaction is complete, is cooled to room temperature, reaction solution is poured into 300g ice water, and control The temperature of the system is less than 10°C;
[0063] Next, the obtained mixed system was filtered, washed with water, and dried, and the yield of the product 2,4 dichloro-3-bromo-5-fluorobenzoic acid was 98%;
[0064] (2) In the reaction flask, add 28.8g of 2,4 dichloro-3-bromo-5-fluorobenzoic acid and 116.2g of tetrahydrofuran, stir to dissolve, and cool to -20~30°C;
[0065] Then, add 50ml 2M i-PrMgCl / THF so...
Embodiment 2
[0071] Prepare 2,6-dichloro-3-fluorobenzaldehyde according to the method described below, and the specific steps are as follows:
[0072] (1) Add 192g of concentrated sulfuric acid with a mass concentration of 95% and 19.2g of 2,4 dichloro-5-fluorobenzoic acid into the reaction flask, heat to 50-55°C, stir to dissolve;
[0073] Then, add 19.7g NBS in batches, then keep the temperature for 5 hours, TLC (ethanol / ethyl acetate=1 / 1) analysis, the raw material reaction is complete, cool to room temperature, the reaction solution is poured into 600g ice water, and control system The temperature is less than 10°C;
[0074] Then, the obtained mixed system was filtered, washed with water and dried, and the yield of the product 2,4 dichloro-3-bromo-5-fluorobenzoic acid was 98%.
[0075] (2) In the reaction bottle, add 28.8g 2,4 dichloro-3-bromo-5-fluorobenzoic acid, 144g tetrahydrofuran, stir to dissolve, cool to 0~-5℃, add dropwise 110ml 2M i-PrMgCl / THF solution, keep warm for half ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com