Method of preparing fluorine-containing alkenyl demoulding intermediate with industrial side product terpinene
A technology for by-producing terpinene and fluorine-containing alkenyl group is applied in the field of preparing fluorine-containing alkenyl-based mold release intermediates, and can solve the problems of difficult separation, difficult economic benefits and the like, and achieves improved processing efficiency, simple and easy reaction operation, The effect of sufficient source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
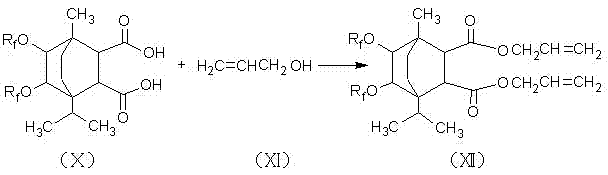
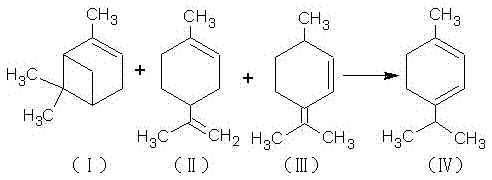
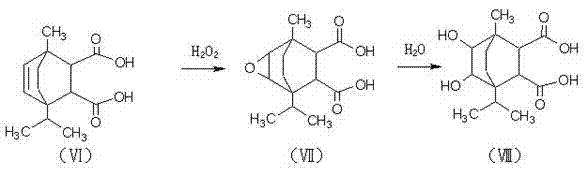
[0026] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 25 grams of terpinene and 1 gram of sulfuric acid, and keep it under reflux at 95°C to 105°C for 1 to 2 hours, after that, add 21 grams of maleic acid , 1.1 grams of phenylsulfonic acid and 0.6 grams of aluminum trichloride, continue to reflux reaction at 110°C to 130°C for 5 hours to generate terpinene maleate addition compound, then add 18 grams of hydrogen peroxide and 5 grams of ice Acetic acid, react at 75°C to 85°C for 4 hours, hydrolyze with 5 times the volume of hot water, then hydrolyze, let stand, take the oil layer, and generate dihydroxy compounds; the generated dihydroxy compounds, 110mL N,N-dihydroxy Methyl sulfoxide and 110 grams of hexafluoropropylene oxide oligomers were placed in a four-necked flask, and 0.8 grams of triethylamine was slowly added to perform a nucleophilic substitution reaction for 8 hours. The product was diluted with water, and 25% ...
Embodiment 2
[0028] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 25 grams of terpinene and 1 gram of sulfuric acid, and keep it under reflux at 90 ° C to 100 ° C for 1 to 2 hours, after that, add 24 grams of maleic acid , 1.2 grams of phenylsulfonic acid, continue to reflux at 120°C to 150°C for 6 hours to generate a terpinene maleate addition compound, then add 21 grams of hydrogen peroxide and 4 grams of glacial acetic acid, and heat at 80°C to 90°C React at ℃ for 2 hours, hydrolyze with 5 times the volume of hot water, let it stand, take the oil layer, and generate bishydroxyl compounds; the generated bishydroxyl compounds, 150mL N,N-dimethylsulfoxide and 130g Slowly add 0.5 g of triethylamine to the propane oligomer in a four-necked flask, and carry out the nucleophilic substitution reaction for 6 hours, dilute the product with water, add 25% mass concentration of sodium hydroxide solution, adjust the pH to about 10, Filter, th...
Embodiment 3
[0030] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 25 grams of terpinene and 1 gram of sulfuric acid, and keep it under reflux at 85°C to 95°C for 1 to 2 hours, and then add 27 grams of maleic acid , 1 gram of phenylsulfonic acid and 1 gram of aluminum trichloride, continue to reflux reaction at 130°C to 160°C for 4 hours to generate terpinene maleate addition compound, then add 24 grams of hydrogen peroxide and 6 grams of ice Acetic acid, react at 70°C to 80°C for 3 hours, hydrolyze with 5 times the volume of hot water, let it stand, take the oil layer, and generate bishydroxyl compounds; the generated bishydroxyl compounds, 130mL N,N-dimethylsulfoxide and 120 grams of hexafluoropropylene oxide oligomer in a four-necked bottle, slowly add 1 gram three Ethylamine, after nucleophilic substitution reaction for 7 hours, dilute the product with water, add 25% mass concentration of sodium hydroxide solution, adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com