Solanesol derivative and preparation method and application thereof
A technology of solanesol derivatives, applied in the field of preparation of solanesol derivatives, can solve problems such as poor permeability of gastrointestinal mucosa, unstable chemical properties, and limited pharmacological activity, and achieve improved physical and chemical stability and good dilution Stability, effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
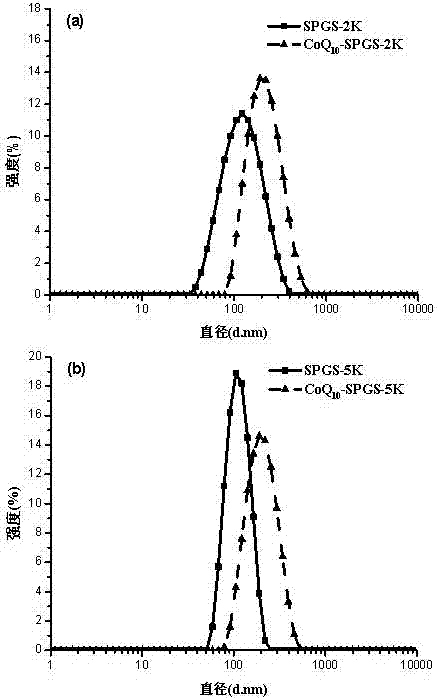
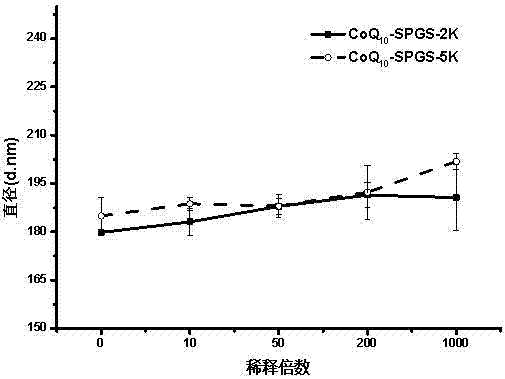
Image
Examples
Embodiment 1
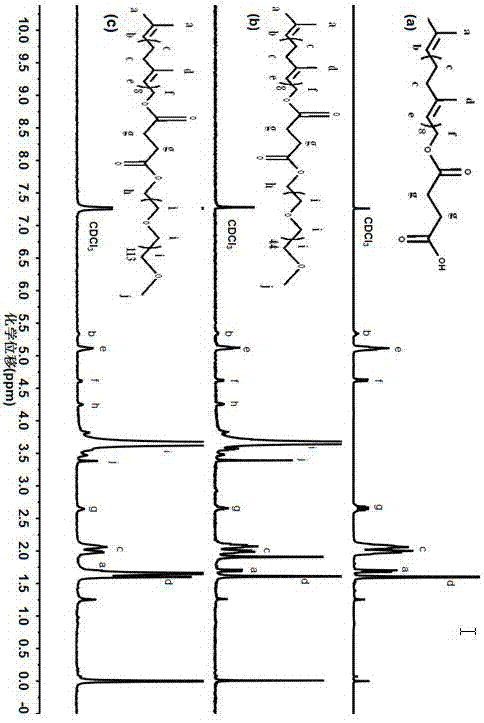
[0032] A solanesol derivative, the derivative structure is as follows:
[0033]
[0034] The synthetic route of above-mentioned solanesol derivative is as follows:
[0035]
[0036] Concrete synthetic steps are as follows:
[0037] (1) Weigh 4.0g SOL (solanesol) and 0.4g DMAP (4-dimethylaminopyridine) and dissolve them in about 25mL anhydrous CH 2 Cl 2 Medium, N 2 Under protection, drop 1.0 g of succinic anhydride and 1.0 mL of triethylamine in 15 mL of DMF into the aforementioned CH 2 Cl 2 Solution, react at room temperature for 48h, after the reaction, the reaction solution is transferred to the funnel, add 30mL distilled water to wash, let it stand, take CH 2 Cl 2 Layers were repeated twice, and the solvent was removed by rotary evaporation. Use petroleum ether:ethyl acetate=1:1 (v / v) as the mobile phase to pass through the silica gel column, TLC monitors the outflow of the product, spins the mobile phase to dryness, and vacuum-dries to obtain the light yellow ta...
Embodiment 2
[0040] A solanesol derivative, the derivative structure is as follows:
[0041]
[0042] The synthetic route of above-mentioned solanesol derivative is as follows:
[0043]
[0044] Concrete synthetic steps are as follows:
[0045] (1) Weigh 4.0g SOL (solanesol) and 0.4g DMAP (4-dimethylaminopyridine) and dissolve them in about 25mL anhydrous CH 2 Cl 2 Medium, N 2 Under protection, drop 1.0 g of succinic anhydride and 1.0 mL of triethylamine in 15 mL of DMF into the aforementioned CH 2 Cl 2 Solution, react at room temperature for 48h, after the reaction, the reaction solution is transferred to the funnel, add 30mL distilled water to wash, let it stand, take CH 2 Cl 2 Layers were repeated twice, and the solvent was removed by rotary evaporation. Use petroleum ether:ethyl acetate=1:1 (v / v) as the mobile phase to pass through the silica gel column, TLC monitors the outflow of the product, spins the mobile phase to dryness, and vacuum-dries to obtain the light yellow ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com