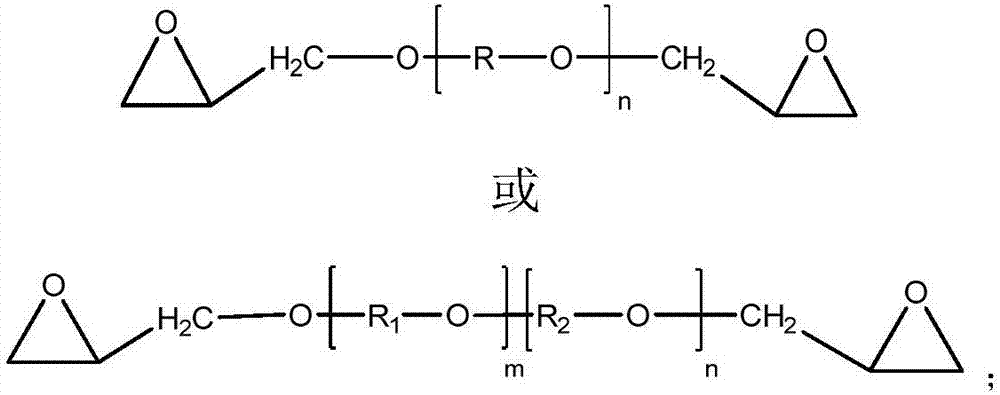
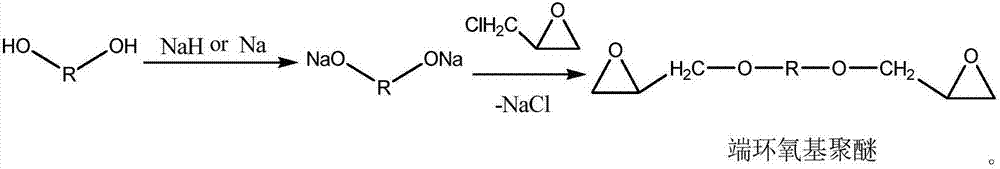
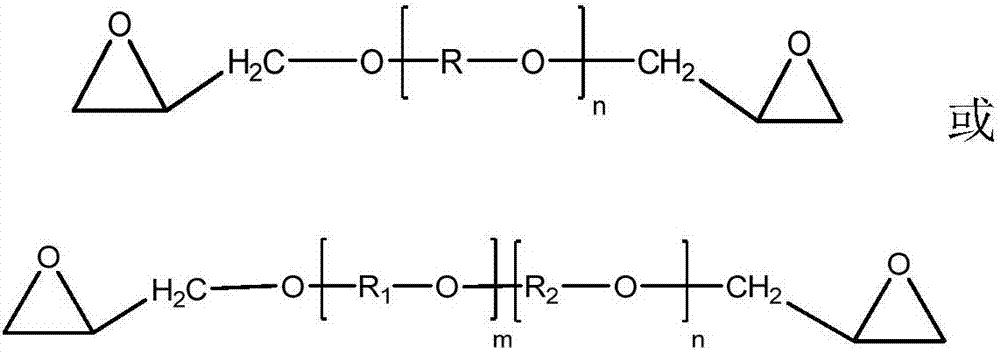
Epoxy-terminated polyether compound as well as preparation method and application thereof
A technology of epoxy-terminated polyether and polyether compounds, which is applied in the field of epoxy-terminated polyether compounds and their preparation, can solve problems such as large safety risks, and achieve the effects of short curing time, high purity and low curing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The epoxy-terminated polyoxyethylene ether of synthetic PEG-6000, concrete method is as follows:
[0030] A 2000 mL four-necked flask was equipped with a mechanical stirrer, a condenser and a thermometer, and 600 g (0.10 mol) of polyethylene glycol (PEG-6000) and 800 mL of dichloromethane were added to the four-necked flask. Add 6.00g of sodium hydride (0.25mol) in batches, control the reaction temperature at 0-10°C, slowly add 23.2g (0.25mol) of epichlorohydrin dropwise, raise the temperature to 40°C and reflux for 4h after the addition, remove the solid by filtration, and wash the filtrate with water To neutrality, dry with anhydrous magnesium sulfate, and remove the dichloromethane solvent by rotary evaporation to obtain 504.2 g of epoxy-terminated polyether of PEG-6000 with a yield of 82.5%.
[0031] The epoxy-terminated polyether of PEG-6000 is used as a binder to prepare a solid propellant, and the specific method is as follows:
[0032] Add 25g PEG-6000 epoxy-te...
Embodiment 2
[0037] The epoxy-terminated polyoxyethylene ether of synthetic PEG-10200, specific method is as follows:
[0038] A 2000 mL four-necked flask was equipped with a mechanical stirrer, a condenser and a thermometer, and 510 g (0.05 mol) of polyethylene glycol (PEG-10200) and 800 mL of dichloromethane were added to the four-necked flask. Add 3.10g of sodium hydride (0.13mol) in batches, control the reaction temperature at 0-10°C, slowly add 12.1g (0.13mol) of epichlorohydrin dropwise, raise the temperature to 40°C and reflux for 4h after the addition, remove the solid by filtration, and wash the filtrate with water To neutrality, dry with anhydrous magnesium sulfate, and remove the dichloromethane solvent by rotary evaporation to obtain 432.6 g of epoxy-terminated polyether of PEG-10200 with a yield of 83.9%.
[0039] The epoxy-terminated polyether of PEG-10200 is used as a binder to prepare a solid propellant, and the specific method is as follows:
[0040] Add 25g PEG-10200 epo...
Embodiment 3
[0045] Synthesize epoxy-terminated polytetrahydrofuran-ethylene oxide copolyether of PET-5500, the specific method is as follows:
[0046] A 1000mL four-necked flask was equipped with a mechanical stirrer, a condenser and a thermometer, and 550g (0.10mol) of polytetrahydrofuran-ethylene oxide copolyether (PET-5500) and 350mL of benzene were added to the four-necked flask. Add 6.20g of sodium hydride (0.25mol) in batches, control the reaction temperature at 0-10°C, slowly add 20.08g (0.26mol) of epichlorohydrin dropwise, raise the temperature to 70±5°C and reflux for 4h after the addition, and remove the solid by filtration. The filtrate was washed with water until neutral, dried over anhydrous magnesium sulfate, and the benzene solvent was removed by rotary evaporation to obtain 466.9 g of epoxy-terminated polyether of polytetrahydrofuran-ethylene oxide copolyether (PET-5500), with a yield of 83.2%.
[0047] The solid propellant is prepared by using epoxy-terminated polytetrah...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com