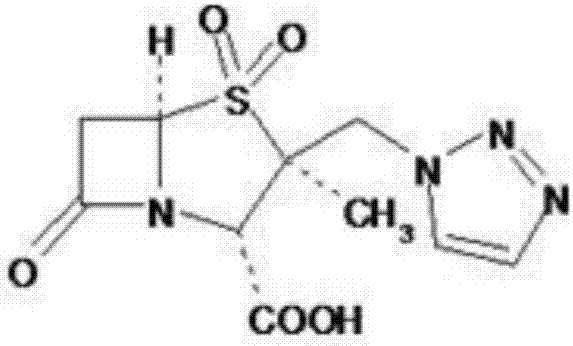
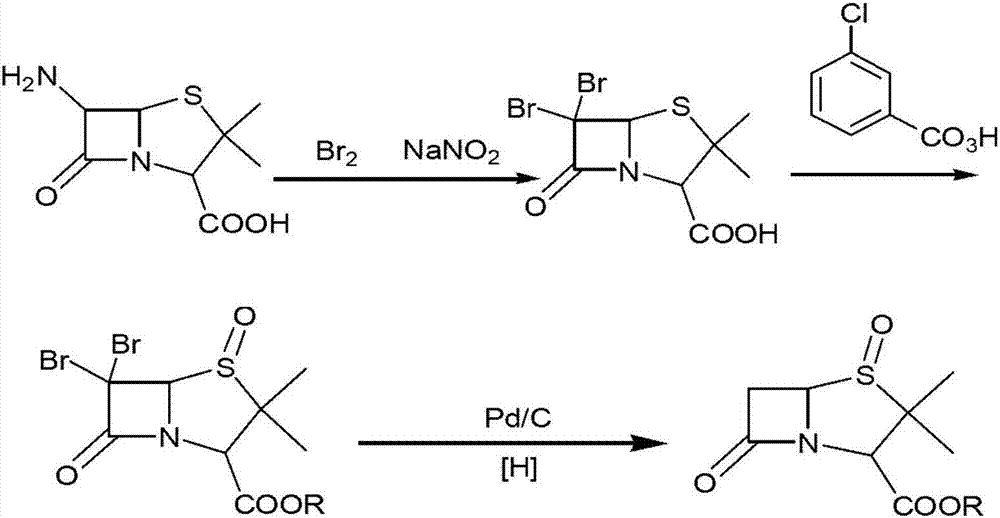
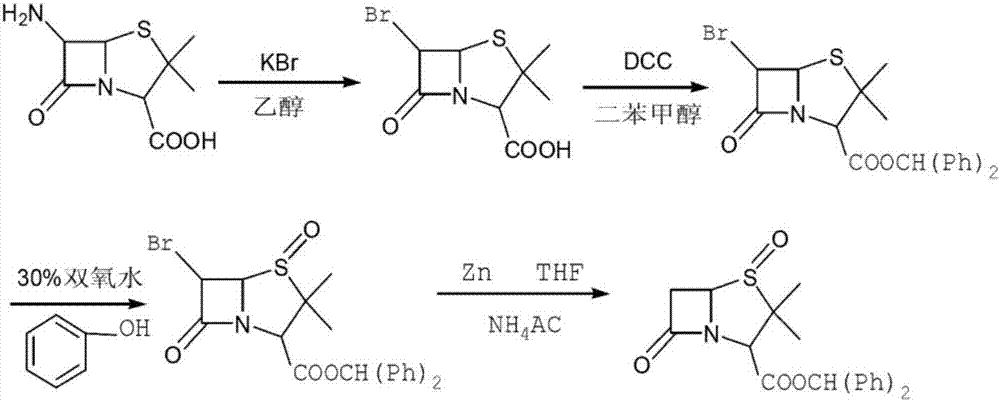
Method for preparing penam sulfoxide acid diphenyl methyl ester which is tazobactam precursor
A technology of diphenylmethyl sulfoxide and aminopenicillanic acid is applied in the field of synthesis of tazobactam pharmaceutical intermediates, and can solve the problems of potential safety hazards, high cost, poor safety and reliability, etc. The effect of reducing the discharge of three wastes and improving process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1), in the reactor of 1500L, add 500L methylene dichloride, 180kg water, drop into 120kg6-aminopenicillanic acid, stir; Then in reactor, dropwise add 48% hydrogen bromide aqueous solution (188kg) and 25% hypochlorite Sodium nitrate solution (153kg), drop the temperature to -10 ℃ and react for 4 hours after the completion of the dropwise addition, point the plate to monitor until the end of the reaction, wash the organic phase to neutrality, obtain 6-bromopenicillanic acid solution, and obtain the 6- The bromopenicillanic acid solution is transferred to the oxidation reactor;
[0040] 2) Add 55kg of potassium bicarbonate to the oxidation reaction kettle and stir for 1.5 hours, then add 102kg of diphenylmethyl chloride and 1kg of tetrabutylammonium bromide, heat up to 30°C, stir for 3 hours, and detect the end point with thin-layer chromatography After the benzhydryl chloride reaction is complete, add 0.5kg sodium tungstate to the oxidation reaction kettle, and start to a...
Embodiment 2
[0043] 1), add 500L dichloromethane, 180kg water in the reactor of 1500L, drop into 120kg6-aminopenicillanic acid, stir well; Then in reactor, dropwise add 50% hydrogen bromide aqueous solution (89.01kg) and 50% Sodium nitrite solution (37.95.kg), drop the temperature to -5 DEG C and react for 3 hours after the completion of the dropwise addition, point the plate to monitor until the end of the reaction, wash the organic phase with water to neutrality, obtain 6-bromopenicillanic acid solution, and obtain The 6-bromopenicillanic acid solution is transferred in the oxidation reactor;
[0044]2) Add 60kg of potassium bicarbonate to the oxidation reaction kettle and stir for 2 hours, then add 102kg of diphenylmethyl chloride and 1kg of tetrabutylammonium bromide, heat up to 20°C, stir and react for 4 hours, and use thin-layer chromatography to detect the end point After the reaction of benzhydryl chloride is complete, add 0.9kg sodium tungstate to the oxidation reaction kettle, an...
Embodiment 3
[0047] 1), add 500L dichloromethane, 180kg water in the reactor of 1500L, drop into 120kg6-aminopenicillanic acid, stir; Then in reactor, dropwise add 50% hydrogen bromide aqueous solution (267kg) and 60% hypodermic acid Sodium nitrate solution (94.875kg), drop the temperature to 0 DEG C and react for 2 hours after completion of the dropwise addition, point the plate to monitor until the end of the reaction, wash the organic phase to neutrality, obtain 6-bromopenicillanic acid solution, and obtain the 6- The bromopenicillanic acid solution is transferred to the oxidation reactor;
[0048] 2) Add 50kg of potassium bicarbonate to the oxidation reaction kettle and stir for 1 hour, then add 102kg of diphenylmethyl chloride and 1.1kg of tetrabutylammonium bromide, heat up to 30°C, stir for 3 hours, and detect with thin layer chromatography At the end point, after the reaction of benzhydryl chloride is complete, add 0.5kg sodium tungstate to the oxidation reaction kettle, and start ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com