Method for preparing N-glycosylated proline aminopeptidase and application
A proline aminopeptidase and glycosylation technology, applied in the field of enzyme engineering, can solve the problems of rapid inactivation, poor decomposition ability of endopeptidase, poor purification efficiency, etc., and achieves sufficient enzymatic hydrolysis reaction and high oligopeptide level. , the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
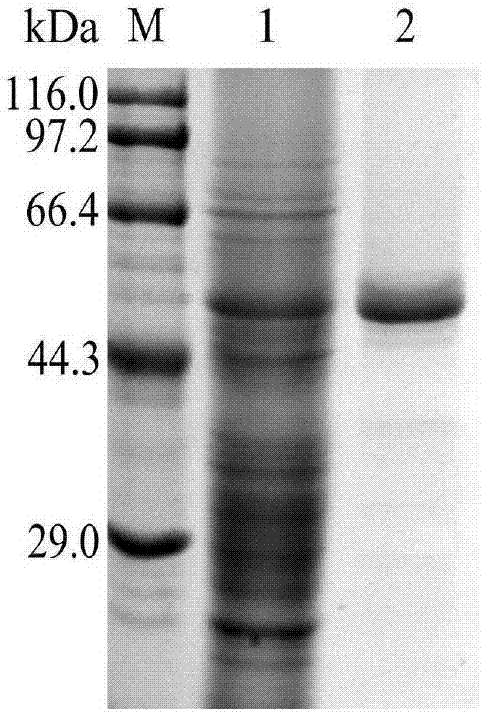
[0049] Embodiment 1 One-step purification of recombinant Pichia proline aminopeptidase
[0050] Design upstream primers (5'-CCGGAATTCATG CATCATCATCATCATCAT GCTGCCAAGCTTGT-3') added a histidine tag to the N-terminus of aminopeptidase by PCR technology, and induced the expression of the recombinant strain for 6 days. After dialysis in solution A for 2 days, the sample was obtained by filtering through a 0.22um filter membrane. After equilibrating Ni-NTA with buffer A, load the sample by draining method, elute for 5 column volumes, and then elute with buffer B containing high concentration of imidazole, collect 5mL in each tube to detect aminopeptidase activity and protein concentration, and carried out SDS-PAGE electrophoresis. The result is as figure 1 As shown, the aminopeptidase obtained by this purification method reaches electrophoretic purity. The protein content and aminopeptidase activity of the purification steps were detected. The results showed that the nickel co...
Embodiment 2
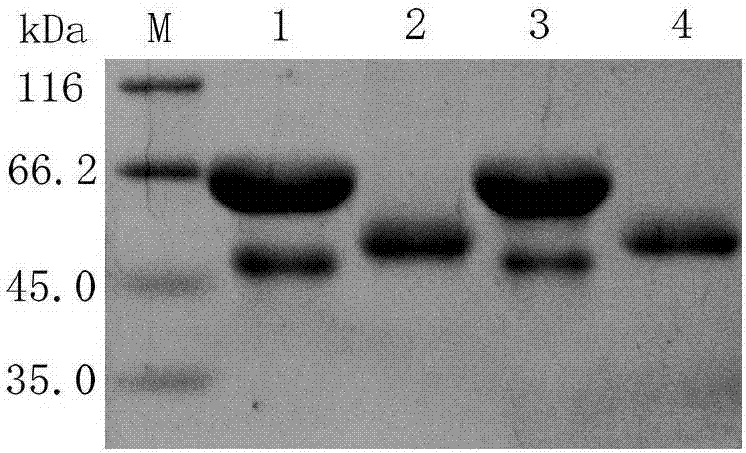
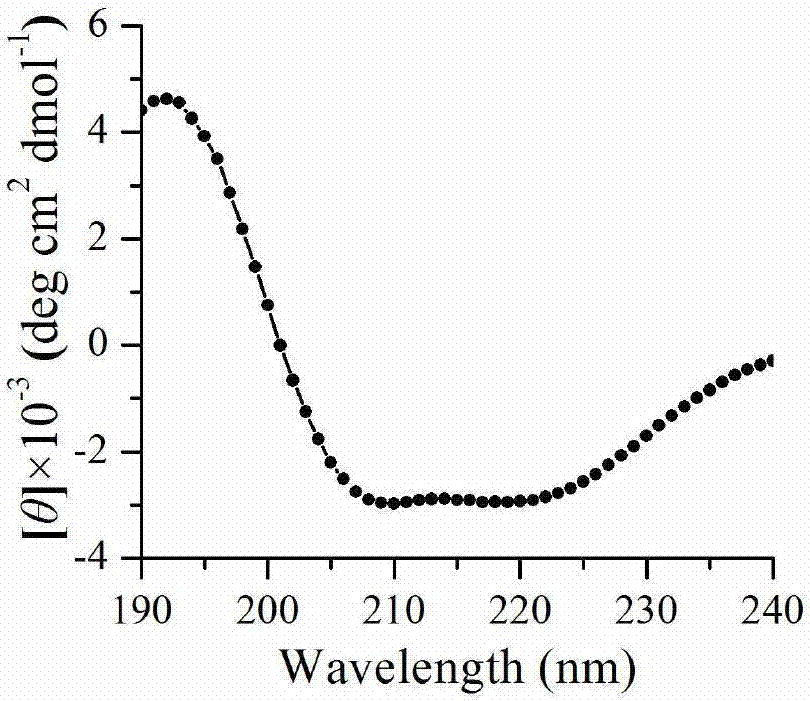
[0052] Example 2 Recombinant proline aminopeptidase N-glycosylation analysis and secondary structure analysis
[0053] The amino acid sequence of proline aminopeptidase was analyzed online to predict its glycosylation site. The analysis showed that there were three potential glycosylation sites, namely NYSR, NWSR and NFSI (28~31, 316~319, 328~331). Take 9uL purified intracellular and extracellular aminopeptidase enzyme solution and mix with 1uL protein denaturation buffer, and then bathe in boiling water for 15min. deglycosylation enzyme Endo H f The reaction was carried out at 37°C for 3 h under the catalysis of the enzyme, and the molecular weight of the protein before and after digestion was analyzed by SDS-PAGE electrophoresis. Such as figure 2 After the deglycosylation treatment shown, the original aminopeptidase band was replaced by a lower molecular weight band, proving that the proline aminopeptidase was completely glycosylated, and the extracellular aminopeptidase...
Embodiment 3
[0055] Example 3 Stability Study of Recombinant Pichia Pastoris Expressing Proline Aminopeptidase
[0056] (1) Optimum reaction temperature and temperature stability of aminopeptidase
[0057] The effect of temperature on the catalytic ability of aminopeptidase was detected with proline-p-nitroaniline as substrate, Figure 4 a shows that its optimum temperature is 60°C. Take 500uL of the purified aminopeptidase enzyme solution and place them in 1.5mL EP tubes, number them and keep them in water baths at 40, 50, and 60°C for 54 hours. Take 20uL every few hours and use standard methods to detect aminopeptidase enzyme activity. It was found that (such as Figure 4 b) Although the remaining enzyme activity is only 5.6% after incubation at the optimal reaction temperature of 60°C for 1 hour, it has good stability at temperatures lower than 60°C. The optimum temperature range of common PAP is 30-45°C (see Table 1). For example, the optimum temperature of PAP derived from D. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com