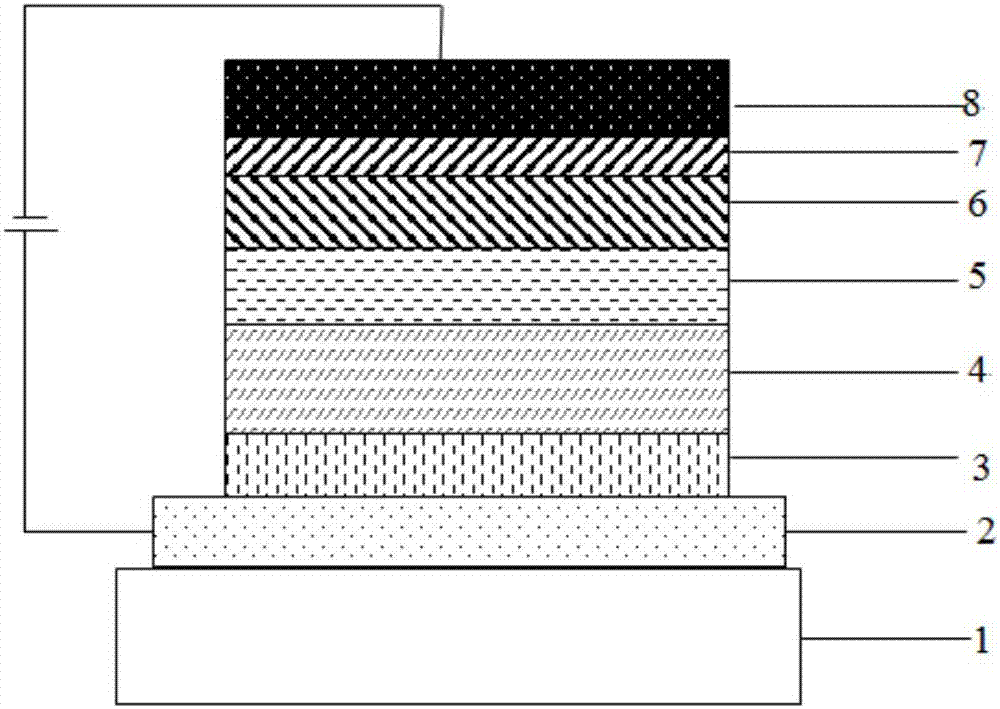
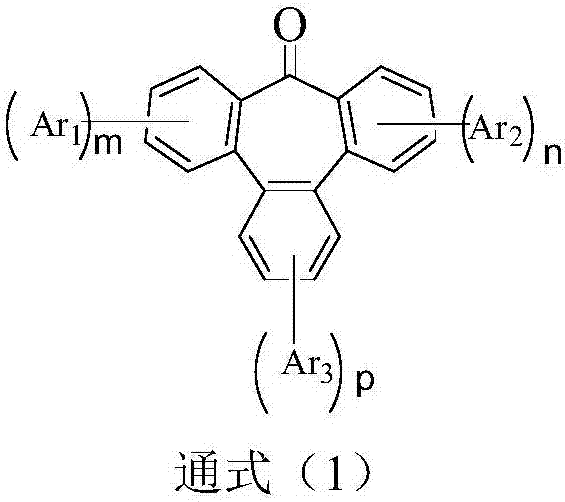
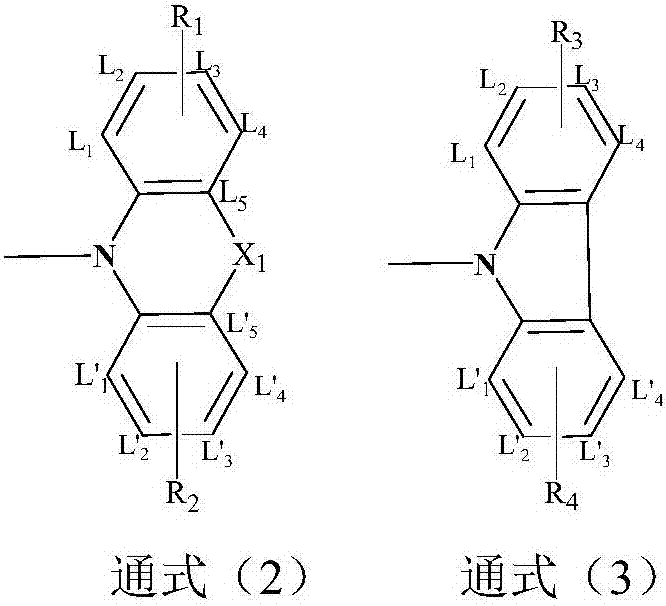
Compound adopting tribenzocycloheptenone as core, and application thereof in OLED devices
A technology of benzocycloheptenone and compound, which is applied in electric solid devices, semiconductor devices, chemical instruments and methods, etc., can solve the problems of limited application and lower triplet energy level, and achieves good optoelectronic performance, high triplet state and the like. state energy levels, the effect of avoiding aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of R structure (shown in general formula 3)
[0047]
[0048] The preparation method of R structure (shown in general formula 3):
[0049] Weigh raw material I o-bromonitro compound, raw material II boric acid, and dissolve it in a mixed solvent of toluene and ethanol with a volume ratio of 2:1, and add potassium carbonate aqueous solution, Pd(PPh 3 ) 4 , reacted at 95-110°C for 10-24 hours, cooled to and filtered the reaction solution, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product; the molar ratio of o-bromonitro compound to boric acid was 1:1.0-3.0; o-bromo The molar ratio of nitro compound to potassium carbonate is 1:1.0~3.0; o-bromonitro compound and Pd(PPh 3 ) 4 The molar ratio is 1:0.006~0.02;
[0050] Weigh the product from the previous step, dissolve it with o-dichlorobenzene, and then add PPh 3 , under an inert atmosphere, react the mixed solution of the above reactant...
Embodiment 2
[0057] Embodiment 2: the synthesis of R structure (shown in general formula 2)
[0058]
[0059] The preparation method of Reaction Formula 3-1 is: Weigh the corresponding raw material 1-o-aminophenol, raw material 2-o-nitrophenol, and iodine, stir and dissolve with diethylene glycol as a solvent, and heat to 250-270°C under an inert atmosphere to react 12 ~ 24 hours, take a sample point plate, after the reaction is over, cool naturally to room temperature, solids are precipitated, filter, take the filter cake and pass through a neutral silica gel column to obtain the intermediate En; the mole of the o-nitrophenol and o-aminophenol The ratio is 1:0.8~2.0; the molar ratio of the o-nitrophenol and iodine is 1:0.05~0.1;
[0060] The preparation method of reaction formula 3-2 is: weigh the corresponding bromide and arylamine with methyl formate, then add Pd 2 (dba) 3 , tri-tert-butylphosphine, sodium tert-butoxide; under an inert atmosphere, react the mixed solution of the ab...
Embodiment 3
[0071] Example 3: Synthesis of halogenated compounds of tribenzocycloheptenone
[0072]
[0073]
[0074] Reaction equation 4-1 specific implementation steps:
[0075] Weigh tribenzocycloheptenone and dissolve it in acetic acid, cool down to 0°C with an ice-salt bath; weigh liquid bromine and dissolve it in glacial acetic acid, and slowly add it dropwise to the acetic acid solution containing tribenzocycloheptenone , after the dropwise addition, rise to room temperature, stir the reaction until the reaction is complete; after the reaction, add lye to the reaction solution for neutralization, extract with dichloromethane, separate layers, take the organic phase and filter, and the filtrate is rotary evaporated under reduced pressure to No fraction, through a neutral silica gel column to obtain the intermediate An; the molar ratio of the tribenzocycloheptenone to liquid bromine is 1:0.5~2;
[0076] Reaction equation 4-2 specific implementation steps:
[0077] Weigh the raw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com