Synthesis method and use of nitrogen-containing functional mesoporous polymer
A technology for nitrogen heterocyclic compounds and polymers is applied in the synthesis and application of nitrogen-containing functionalized mesoporous polymers, which can solve the problems of poor catalytic effect, poor circulation ability, long synthesis period and the like, and achieves a simple and feasible preparation method. control, easy separation, and short synthesis period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
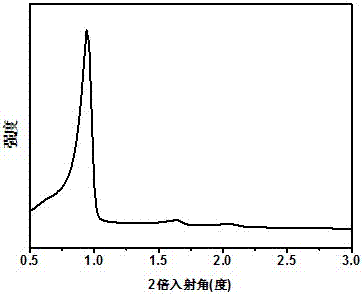
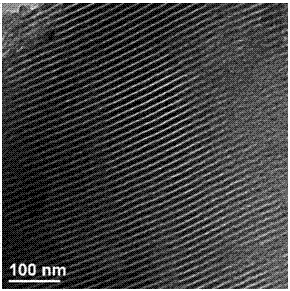
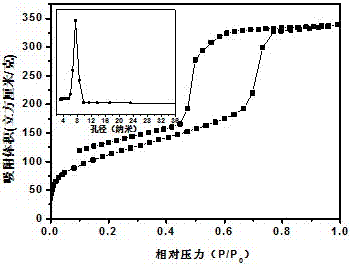
Image
Examples
Embodiment 1
[0025] a, the preparation of intermediates
[0026] After mixing 4.3g sodium hydride and 5mL tetrahydrofuran, add dropwise to the mixture of 0.1mol imidazole and 40mL tetrahydrofuran, stir and mix for 40min, then add 0.1mol 3-chloromethyl anisole, reflux at 70°C for 24h, and react The product was washed with 100 mL of deionized water and extracted with 100 mL of dichloromethane, then the organic phase was washed twice with deionized water, once with saturated brine and dried over anhydrous sodium sulfate, spin-dried with dichloromethane and obtained 15.3 g by column chromatography The intermediate is 3-imidazole methyl anisole.
[0027] b, the preparation of nitrogen source monomer
[0028] Take 10 g of the above-prepared 3-imidazole methyl anisole and add it to 50 mL of hydrobromic acid, reflux at 120 ° C for 24 h for demethylation, and use Na 2 CO 3 Neutralize hydrobromic acid, add saturated NaHCO when the pH is almost 7 3 Adjust the pH of the reaction solution to 7.5, t...
Embodiment 2
[0037]0.15g of the functionalized mesoporous polymer prepared in the above example 1, 0.1g of potassium iodide, 1.74g of propylene oxide and 0.1g of internal standard biphenyl were placed in a 25mL stainless steel autoclave, and the pressure was 1MPa of carbon dioxide gas, carry out the cycloaddition reaction at 70°C for 3 hours, then place the reactor in an ice bath to cool, and slowly release excess CO 2 , the reaction solution was diluted with DMF solution and detected by gas chromatography, the product obtained was propylene carbonate, the conversion rate was 70.0%, and the selectivity was 99.5%.
Embodiment 3
[0039] 0.3g of the functionalized mesoporous polymer prepared in the above example 1, 0.1g of potassium iodide, 1.74g of propylene oxide and 0.1g of internal standard biphenyl were placed in a 25mL stainless steel autoclave, and a pressure of 1MPa was introduced into the carbon dioxide gas, carry out the cycloaddition reaction at 70°C for 3 hours, then place the reactor in an ice bath to cool, and slowly release excess CO 2 , the reaction solution was diluted with DMF solution and detected by gas chromatography, the product obtained was propylene carbonate, the conversion rate was 87.0%, and the selectivity was 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com