A drug-loaded hydrogel with dual responsiveness to enzyme and temperature and its preparation method and application
A technology of responsiveness and potion, applied in the direction of medical formula, microcapsules, capsule delivery, etc., can solve the problems of collapse dissolution, loss of drug molecules, toxic and side effects of the body, etc., to prolong the action time, reduce the pain of patients, and operate simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
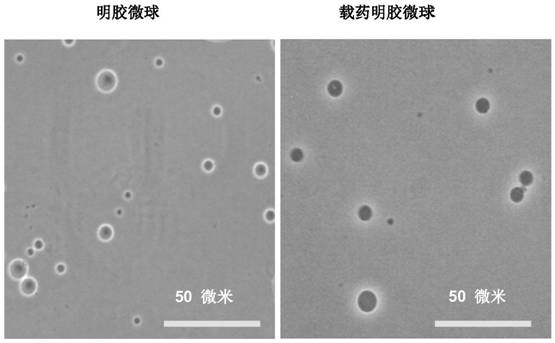
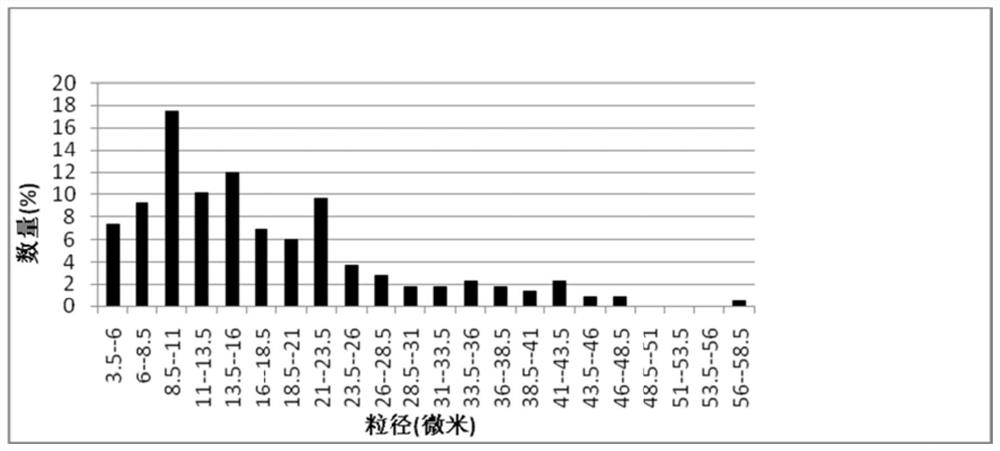
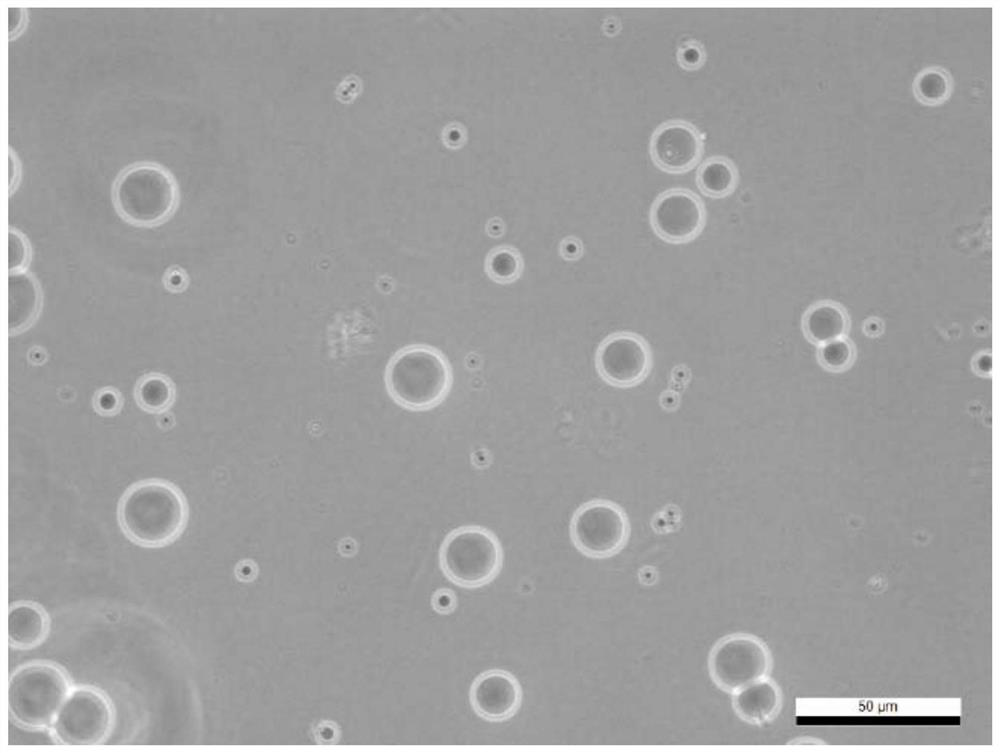
[0038] In the preparation process of the carrier, the inventor tried to select natural materials or clinically approved materials as raw materials, such as gelatin, chitosan, modified starch, etc. The combination of these raw materials and nano-sized drugs can ensure that the drug has better clinical safety; and it is finally determined that when the particle size of the carrier is 1-20 μm, it can be enriched around the lesion and not enter the blood in the case of local application. circulatory system.
[0039] Gelatin is an analogue of the extracellular matrix obtained from acid-denatured pig skin. Due to the evolutionary conservation of the extracellular matrix of vertebrates (even including some invertebrates) (for example, collagen maintains the structural pattern of Gly-X-Y from lower animals to humans), the possibility of causing immune rejection in organisms lower. Gelatin is a denatured extracellular matrix, which can be used as a substrate for matrix metalloprotein...
Embodiment 1
[0087] (1) Nano-drugs: Dissolve curcumin as a drug in tetrahydrofuran to form a drug solution with a concentration of 0.1 mg / ml, stir physiological saline at a speed of 2000 rpm, then slowly add the drug solution therein, and stir evenly After 30 minutes, a curcumin nanoparticle solution was formed; after standing still for 24 hours, the THF was removed by rotary evaporation, and then freeze-dried at a vacuum degree of 9 Pa at a temperature lower than -50° C. to obtain curcumin nanoparticles. The average particle diameter of curcumin nanoparticles is 50-100nm.
[0088] (2), forming drug-loaded gelatin microspheres: adding gelatin to double-distilled water, stirring at 55° C. at 800 rpm to obtain a 10wt% gelatin solution; slowly adding the curcumin nanoparticles obtained in step (1) to the gelatin solution, fully Stir for 2h to form an aqueous phase. Take 4.5ml of liquid paraffin, add 45μl of Span-80 (Span-80), stir evenly at 55°C and 800rpm to form an oil phase. Slowly and e...
Embodiment 2
[0091] (1), nano-ization of medicine: dissolving curcumin as medicine in ethanol to form a medicine solution with a concentration of 5 mg / ml, stirring physiological saline under the condition of 700 rpm, then slowly adding medicine solution therein, and stirring evenly for 2 hours , forming a curcumin nanoparticle solution; after standing still for 24 hours, the ethanol was removed by rotary evaporation, and then freeze-dried at a vacuum degree of 7 Pa at a temperature lower than -50° C. to obtain curcumin nanoparticles. The average particle diameter of curcumin nanoparticles observed under scanning electron microscope is 100nm.
[0092] (2), forming drug-loaded gelatin microspheres: adding gelatin to double-distilled water, stirring at 55°C at 1500rpm to obtain a 10wt% gelatin solution; slowly adding the curcumin nanoparticles obtained in step (1) to the gelatin solution, fully Stir for 0.5h to form an aqueous phase. Take 4.5ml of liquid paraffin, add 45μl of Span-80 (Span-8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com