Method for preparing unsaturated alcohol through selective hydrogenation of alpha,beta-unsaturated aldehyde
A selective hydrogenation and unsaturated technology, applied in the field of selective hydrogenation of β-unsaturated aldehydes to unsaturated alcohols, α, can solve the problems of low conversion rate of raw materials and product selectivity, small production capacity, and difficult separation of catalysts and products , achieve high raw material conversion rate and unsaturated alcohol selectivity, save energy consumption and save separation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
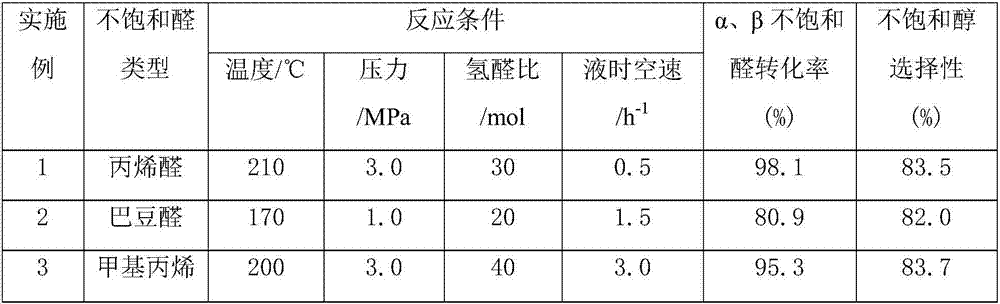
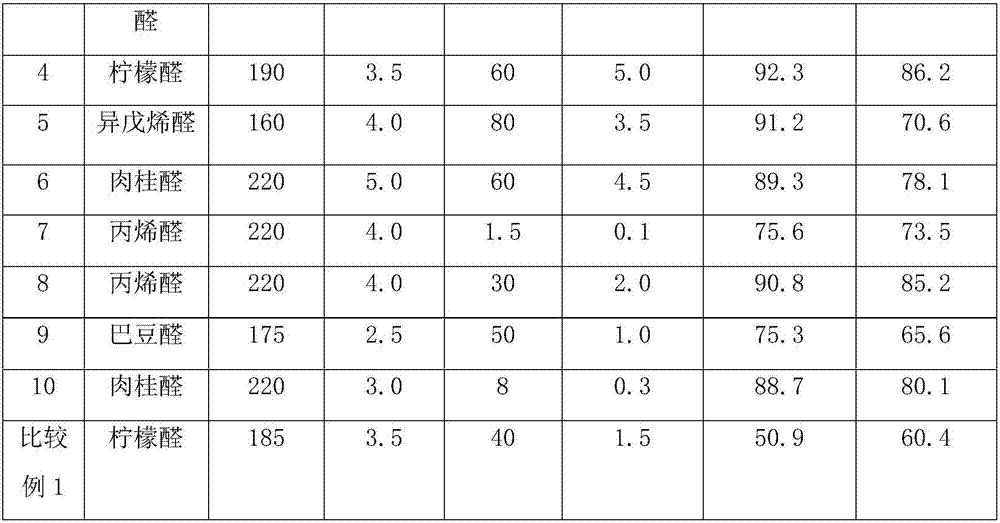
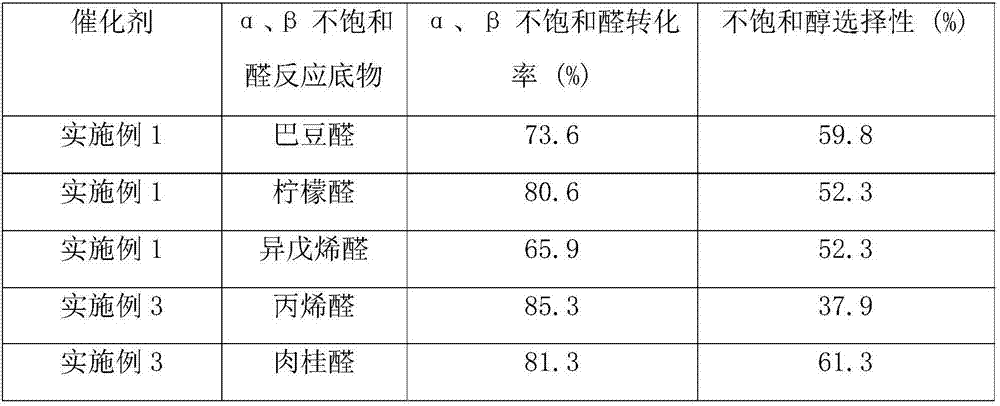
[0022] Weigh a certain amount of MgO carrier, prepare an aqueous solution containing a certain amount of copper nitrate and lanthanum nitrate, the concentrations are 0.5M and 0.22M respectively, soak at room temperature for 12h, and then stir and dry at 100°C. The obtained sample was dried at 120° C. for 12 h, and then calcined at 400° C. for 8 h to prepare precursor 1 . Prepare palladium chloride and rhodium chloride aqueous solutions with concentrations of 0.1M and 0.01M respectively, add 0.1M ethylene glycol and stir, impregnate precursor 1 at room temperature for 12 hours, then stir and dry at 90°C, and dry the obtained samples in Dry at 120°C for 12h, and bake at 500°C for 4h. The catalyst is shaped into cylindrical particles (diameter 3.0mm, height 3.0mm), 100 grams of catalyst is packed into a fixed bed reactor, and reduced at 300°C for 10h (3.0MPa, 2000h -1 ). The supported catalyst 0.05%Rh-7%Cu-1.1%Pd-3%La / MgO was obtained.
[0023] After adding the acrolein into t...
Embodiment 2
[0027] A certain amount of copper nitrate and zirconium nitrate was formulated into a solution, and co-precipitated with aqueous sodium carbonate solution at a precipitation temperature of 50°C, then filtered, dried at 120°C for 8 hours, and calcined at 400°C for 4 hours. Prepare a certain aqueous solution of chloroauric acid and indium nitrate, the concentrations are 0.1M and 0.01M respectively, add 0.5M sodium dodecylbenzene sulfonate, soak at room temperature for 6h, then dry at 80°C, and dry at 120°C for 12h , 380 ℃ roasting 4h. The catalyst is shaped into a clover shape (diameter 3.0mm, length 3.0-5.0mm), 150 grams of catalyst is packed into a fixed-bed reactor, and reduced at 280°C for 6h (1.0MPa, 500h -1 ), to obtain a supported catalyst 5%Cu-1%Au-0.1%In / ZrO 2 .
[0028] The purity of the product obtained by rectification in the rectification tower is ≧99.5%.
Embodiment 3
[0030] Weigh a certain amount of tetrabutyl titanate, dissolve it in a small amount of ethanol, and then dissolve it in a certain amount of water. Weigh a certain amount of rhodium chloride, ruthenium chloride and manganese nitrate aqueous solution to prepare a nitrate solution. Add the nitrate solution into the tetrabutyl titanate solution, stir at 80°C, and add hydrochloric acid dropwise. After the sol was formed, it was aged at room temperature for 24 h. The gel was formed at 80°C, dried at 120°C for 12 hours, and baked at 450°C for 3 hours. The catalyst is shaped into a clover shape (diameter 3.0mm, length 3.0-5.0mm), 75 grams of catalyst is packed into a fixed bed reactor, and reduced at 250°C for 6h (2.5MPa, 3000h -1 ), to obtain a supported catalyst 0.1%Rh-2%Ru-4%Mn / TiO 2 .
[0031] The purity of the product obtained by rectification in the rectification tower is ≧99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com