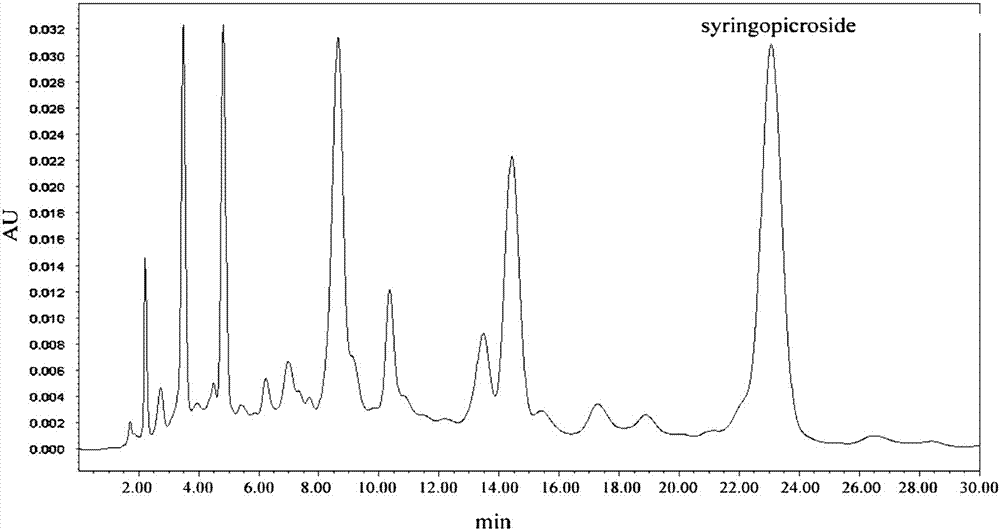
Preparation methods and applications of high-purity total iridoid glycoside extract from folium syringae and preparations of high-purity total iridoid glycoside extract from folium syringae
A total iridoid glycosides, high-purity technology, applied in the direction of pill delivery, pharmaceutical formulations, antiviral agents, etc., to achieve the effects of short filling time, good application prospects, and large sample load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
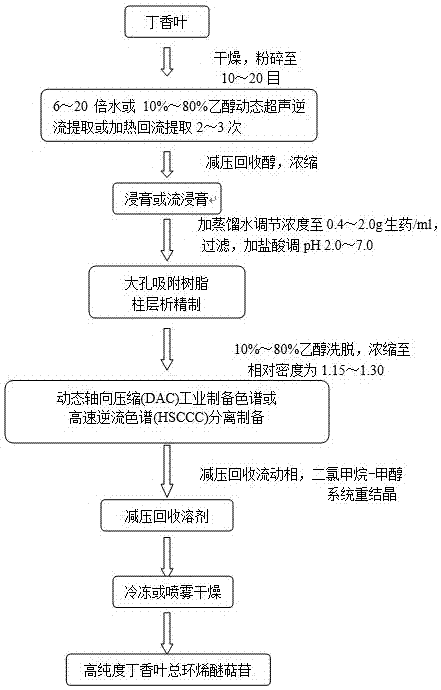
[0019] Example 1 High-purity clove leaves total iridoid glycosides extract was prepared by dynamic ultrasonic countercurrent extraction combined with macroporous adsorption resin column chromatography and dynamic axial compression industrial preparation chromatographic separation technology:
[0020] Grind 10 kg of clove leaves to 20-30 meshes, add 8 times the amount of ethanol with a concentration of 70% by volume, extract continuously at a frequency of 30 kHz and ultrasonic power of 20 kW for 3 times, 40 min each time, filter, and combine the extracts. Recover the extraction solvent under reduced pressure, properly concentrate to a concentration of 0.6 g crude drug / ml, add 1 mol / L hydrochloric acid to adjust the pH value to 4, and pass through the HPD650 macroporous adsorption resin chromatography column at a flow rate of 2 BV / h. The ratio of solid matter to dry resin is 1:6, and the ratio of diameter to height is 1:8. First wash the resin column bed with 4 BV of distilled wa...
Embodiment 2
[0021] Example 2 Heated reflux extraction combined with macroporous adsorption resin column chromatography and high-speed countercurrent chromatography to prepare high-purity clove leaves total iridoid glycosides extract:
[0022] Add 15 times the amount of ethanol with a volume percentage concentration of 80% to 2 kg of clove leaves crushed to 20-30 mesh, heat and reflux for extraction three times, each time for 1.5 h, filter, combine the extracts, and concentrate to a concentration of 1.0 g Crude drug / ml, after filtering, add 1 mol / L hydrochloric acid to adjust the pH value to 4-5, pass through the D101 macroporous adsorption resin chromatography column at a flow rate of 3 BV / h, and the ratio of the sample solution to the dry resin is 1:8, the diameter-to-height ratio is 1:10, first wash the resin with 6 BV of water, the elution flow rate is 4 BV / h, discard the water eluent; then elute with 60% methanol by volume, The elution flow rate is 3 BV / h, and the eluent is decompress...
Embodiment 3
[0023] Example 3 Heated reflux extraction combined with macroporous adsorption resin column chromatography and dynamic axial compression industrial preparation chromatographic separation technology to prepare high-purity clove leaves total iridoid glycosides extract:
[0024] 5 kg of clove leaves are moderately crushed, add 10 times the amount of 60% methanol, and extract 3 times at a frequency of 30 kHz and ultrasonic power of 20 kW, 0.5 h each time, filter, combine the extracts, recover methanol under reduced pressure, and add water to adjust the concentration to 1.5 g crude drug / ml, add 1 mol / L hydrochloric acid to adjust the pH value to 6, and pass through the HPD400 macroporous adsorption resin chromatography column twice at a flow rate of 2BV / h. The ratio of the loading sample solution to the dry resin is 1:9, the diameter-to-height ratio is 1:8, first wash the resin with 4 BV of water, the elution flow rate is 2BV / h, discard the water eluent; then elute with 70% ethanol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com