Diclofenac sodium multi-unit sustained-release micro-pill
A technology of diclofenac sodium and sustained-release micropills, which is applied in pill delivery, sugar-coated pills, coatings, etc., can solve the problems of micropill rupture, adverse safety hazards, and high production costs, so as to facilitate large-scale production and avoid sudden Release effect, effect of flexible dosing regimen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
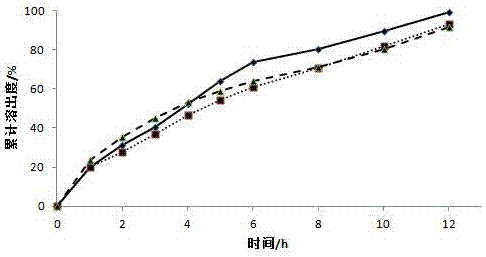
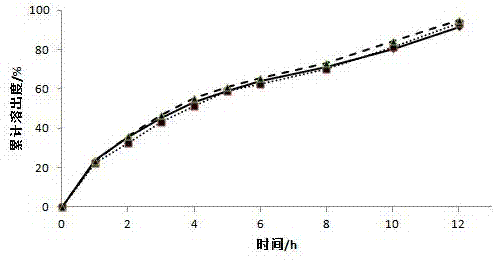
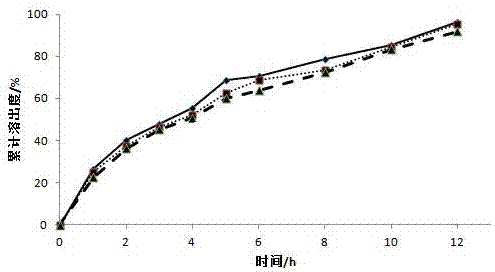
[0032] Weigh 60g of diclofenac sodium, 20g of microcrystalline cellulose, and 200g of lactose, mix them through an 80-mesh sieve, transfer them to a wet granulator, adjust the parameters, add a 1% aqueous solution of hypromellose E15 as a binder to make soft Diclofenac sodium drug-containing pellets were prepared by extrusion and spheronization, wherein the extrusion screen aperture was 0.5mm, the extrusion speed was 20r / min, the spheronization speed was 1000r / min, the fluidized bed was dried at 40°C, and 30-40 mesh drug-containing pellets were sieved. The pellets are for use.
[0033] Place the screened diclofenac sodium pellets in a fluidized bed, prepare a coating solution, and adjust the air intake to 130m 3 / h, fan frequency 26.5HZ, material temperature 28 ℃, coating solution flow rate 2rpm, control coating weight gain 27%, obtained diclofenac sodium sustained-release pellets.
[0034] Coating solution ratio:
[0035]
[0036] Weigh 30g of diclofenac sodium pellets, ...
Embodiment 2
[0038] Weigh 60g of diclofenac sodium, 150g of microcrystalline cellulose, and 90g of lactose, mix them through an 80-mesh sieve, transfer them to a wet granulator, adjust the parameters, and add a 1% aqueous solution of hypromellose E15 as a binder to make soft Diclofenac sodium drug-containing pellets were prepared by extrusion and spheronization, wherein the extrusion screen aperture was 0.5mm, the extrusion speed was 20r / min, the spheronization speed was 1000r / min, the fluidized bed was dried at 40°C, and 30-40 mesh drug-containing pellets were sieved. The pellets are for use.
[0039] Place the screened diclofenac sodium pellets in a fluidized bed, prepare a coating solution, and adjust the air intake to 130m 3 / h, fan frequency 26.5HZ, material temperature 28°C, coating solution flow rate 2rpm, coating weight gain of 29% was controlled to prepare diclofenac sodium sustained-release pellets.
[0040] Coating solution ratio:
[0041] Sustained release material ...
Embodiment 3
[0044] Weigh 60g of diclofenac sodium, 90g of microcrystalline cellulose, and 150g of lactose, mix them through an 80-mesh sieve, transfer them to a wet granulator, adjust the parameters, add a 1% aqueous solution of hypromellose E15 as a binder to make soft Diclofenac sodium drug-containing pellets were prepared by extrusion and spheronization, wherein the extrusion screen aperture was 0.5mm, the extrusion speed was 20r / min, the spheronization speed was 1000r / min, the fluidized bed was dried at 40°C, and 30-40 mesh drug-containing pellets were sieved. The pellets are for use.
[0045] Place the screened diclofenac sodium pellets in a fluidized bed, prepare a coating solution, and adjust the air intake to 130m 3 / h, fan frequency 26.5HZ, material temperature 28 ℃, coating solution flow rate 2rpm, control coating weight gain 25%, obtained diclofenac sodium sustained-release pellets.
[0046] Coating solution ratio:
[0047] Sustained release material
[0048] Weigh ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com