Graphene combined multilayered perforated spheroidic lithium manganese oxide electrode material and lithium ion battery prepared therefrom
A spherical lithium manganate and graphene composite technology, applied in the direction of positive electrode, battery electrode, secondary battery, etc., can solve the problem that the nanomaterial activity accelerates the dissolution of the material, reduces the stability, and the cycle performance and rate of lithium manganate cannot be obtained. Significant improvement and other problems, to achieve the effect of enhancing cycle stability, enhancing electronic conductivity, improving ion diffusivity and cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
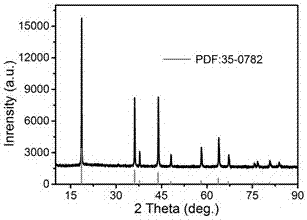
[0040]The synthesis steps of the graphene composite multilayer porous spherical lithium manganate used in the present invention are as follows: Weigh 0.25 g of cetyltrimethylammonium bromide (CTAB) and 11.85 g of ammonium bicarbonate and dissolve them in 250 mL of deionized Mixed solution 1 was formed in water, and 2.54 g of manganese sulfate was weighed and dissolved in 250 mL of deionized water to form mixed solution 2. Add the mixed solution 2 dropwise to the mixed solution 1 in an oil bath at 50°C for 30 minutes, adjust the pH to 7.5, keep the constant pH in the oil bath for 30 minutes, and leave the turbid solution for one day, then wash with water and centrifuge Dry in an oven at 60°C to obtain primary powder of manganese carbonate. The primary powder was calcined in a muffle furnace at 710°C to obtain the precursor manganese trioxide. Take 0.084 g of LiOH·H 2 O and 0.3 g of manganese trioxide were placed in a mortar, and 1 mL of absolute ethanol was used as a solvent....
Embodiment 2
[0044] The preparation of the graphene composite multilayer spherical lithium manganate material with holes is the same as that in Example 1.
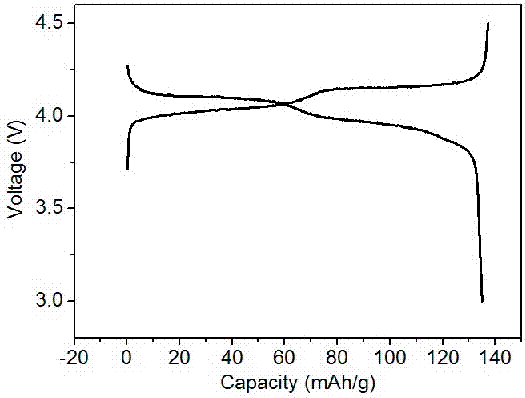
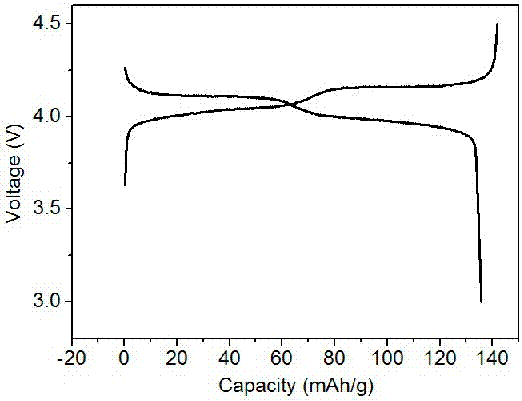
[0045] The prepared graphene composite multilayer porous spherical lithium manganese oxide material is used as the positive electrode active material, and commercial graphite is used as the negative electrode active material. Mixing, using 1-methyl-2-pyrrolidone as a dispersant, mixing the above mixture evenly, adjusting it into a slurry, coating it on aluminum foil and copper foil respectively, drying and cutting at 60°C to obtain the corresponding positive and negative pole pieces ( The capacity of the negative electrode is much larger than the capacity of the cut positive electrode sheet), the electrode sheet and the lithium sheet are separated by Celgard 2500 polypropylene microporous membrane, and 1 M LiPF is used 6 Soluble in EC: DMC (mass ratio 1:1) as the electrolyte, stainless steel shell as the shell, assembled into a CR2016 ...
Embodiment 3
[0047] The graphene composite multilayer porous spherical lithium manganate material prepared in Example 1 was used as the positive electrode active material, and the positive electrode material was mixed with acetylene black and polyvinylidene fluoride in a mass ratio of 80:10:10, and 1-methyl - 2-Pyrrolidone is used as a dispersant. Mix the above mixture evenly to form a slurry and apply it on an aluminum foil. After drying at 60°C, cut it to obtain the positive electrode sheet. The lithium sheet is the negative electrode (the capacity of the negative electrode is much larger than the capacity of the positive electrode sheet). The positive pole piece and the lithium piece are separated by whatman glass fiber separator, using 1 MLiPF 6 Soluble in EC: DMC (mass ratio 1:1) as the electrolyte, stainless steel shell as the shell, assembled into a CR2025 button battery, the lithium-ion battery assembled in the above process has a potential range of 3.0 V-4.5 V at room temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com