Novel synthesis method of aromatic 5-chloro-2-fluoro-4-(trifluoromethyl) aniline hydrochloride containing trifluoromethyl intermediate
A technology of aniline hydrochloride and synthesis method, applied in new synthesis field, can solve problems such as low yield, and achieve the effects of high yield, wide applicability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 2 L three-necked flask, dissolve 68.58 g (270 mmol) iodine element and 83.7 g (270 mmol) silver sulfate in 1.5 L absolute ethanol solution, and stir and cool the reaction solution to 0-5°C. 39.15 g (270 mmol) of 5-chloro-2-fluoroaniline was quickly added to the reaction solution. During the addition, the reaction temperature was kept at 0-5°C, and then slowly raised to room temperature for 1 hr. TLC (petroleum ether / ethyl acetate=1 / 8) traced the disappearance of the starting material, quenched with water (250 mL), and extracted with ethyl acetate (3×250 mL). The organic phase was washed with saturated sodium thiosulfate solution (35.6%) (3×300 mL), and dried over anhydrous sodium sulfate. The solvent was removed, and the crude product (about 80 g) was purified by column chromatography (gradient elution procedure: petroleum ether, petroleum ether / ethyl acetate=3 / 10), and the eluent was placed at room temperature, evaporated and concentrated to obtain a white Solid ...
Embodiment 2
[0021] In a 2 L three-necked flask, 54.2 g (200 mmol) of compound A and 55.3 mL (400 mmol) of triethylamine were dissolved in 1.5 L of dry dichloromethane solution, and the reaction solution was stirred and cooled to 0-5 °C. Add 17.1 mL (240 mmol) of acetyl chloride to the reaction solution slowly dropwise. During the addition, the reaction temperature is kept at 0-5°C, then slowly rise to room temperature, and react for 1 hr. TLC (petroleum ether / ethyl acetate=1 / 10) traced the disappearance of the starting material, quenched with water (250 mL), and extracted with dichloromethane (3×200 mL). The organic phase was washed with saturated sodium chloride solution (3×200 mL), dried over anhydrous sodium sulfate, and concentrated by evaporation to obtain 60.0 g of compound B as a white solid (yield 96%).
[0022] In a 1 L three-necked flask, 31.3 g (100 mmol) of compound B, 89.5 g (500 mmol) of hexamethylphosphoric triamide and 29.2 g (150 mmol) of copper iodide were dissolved in 0...
Embodiment 3
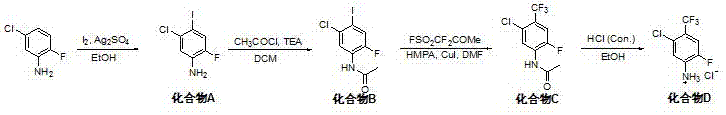
[0024] In a 500 mL three-neck flask, 12.75 g (50 mmol) of compound C was dissolved in 200 mL absolute ethanol solution, 100 mL (600 mmol) of 6 mol / L hydrochloric acid was added at room temperature, and the reaction solution was heated to reflux for 3 hr. Then slowly warm to room temperature. TLC (petroleum ether / ethyl acetate=1 / 7) traced the disappearance of the raw material, and the reaction solution was evaporated and concentrated at low temperature to obtain 11.2 g of compound D as a brown solid (yield 90%). 1 H NMR (400 MHz, DMSO-d6) δ: 6.92 ~7.06 (4 H, m), 7.42 ~7.50 (1 H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com