A kind of aspartic acid ornithine injection and preparation method thereof
A technology of ornithine aspartate and injection, which is applied in the field of medicine, can solve the problems of increased ornithine content, unstable solution quality, complicated preparation process, etc., and achieves reduction of hydrolysis and poor physical and chemical stability. , the effect of high drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Ornithine Aspartate Injection is prescribed as:
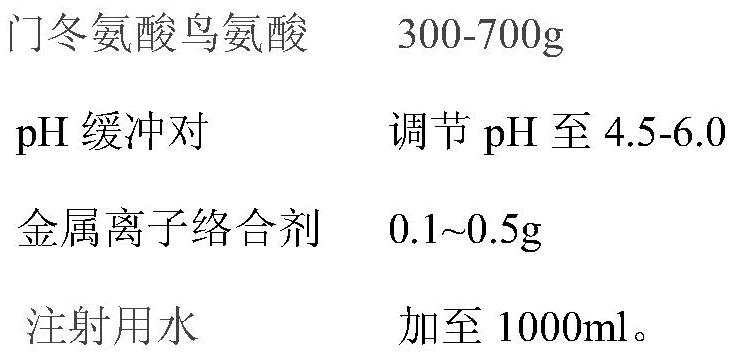
[0039] Include the following weight components in the described injection of every 1000ml:
[0040]
[0041] The preparation process of ornithine aspartic acid injection is as follows:
[0042](1) Pre-purification of ornithine aspartate: Dissolve the raw material of ornithine aspartate in purified water, stir to dissolve and add activated carbon for decolorization. After filtration, the filtrate is heated to 45°C, slowly added methanol dropwise, and then cooled Crystallize at room temperature, filter, wash the filter cake with methanol, and dry under vacuum at 45°C to obtain pre-purified ornithine aspartate;
[0043] (2) In a 2L configuration container, add 50% of the prescribed amount of water for injection, pass nitrogen to saturation, slowly add the prescribed amount of metal ion complexing agent disodium edetate, stir and dissolve;
[0044] (3) use citric acid-sodium citrate buffer to adjust the pH 4.5 of step (...
Embodiment 2
[0048] Ornithine Aspartate Injection is prescribed as:
[0049] Include the following weight components in the described injection of every 1000ml:
[0050]
[0051] The preparation process of ornithine aspartic acid injection is as follows:
[0052] (1) Pre-purification of ornithine aspartate: Dissolve the raw material of ornithine aspartate in purified water, stir to dissolve and add activated carbon for decolorization. Crystallize at room temperature, filter, wash the filter cake with ethanol, and dry under vacuum at 65°C to obtain pre-purified ornithine aspartate;
[0053] (2) In a 2L configuration container, add 50% of the prescribed amount of water for injection, pass nitrogen to saturation, slowly add the prescribed amount of metal ion complexing agent calcium sodium edetate, stir and dissolve;
[0054] (3) Use potassium hydrogen phthalate-sodium hydroxide buffer to adjust the pH 6.0 of step (2) Chinese medicinal liquid;
[0055] (4) After the medicinal solution i...
Embodiment 3
[0058] Ornithine Aspartate Injection is prescribed as:
[0059] Include the following weight components in the described injection of every 1000ml:
[0060]
[0061] The preparation process of ornithine aspartic acid injection is as follows:
[0062] (1) Pre-purification of ornithine aspartate: Dissolve the raw material of ornithine aspartate in purified water, stir to dissolve and add activated carbon for decolorization. After filtration, the filtrate is heated to 50°C, and polyethylene glycol is slowly added dropwise. Then cool to room temperature for crystallization, filter, wash the filter cake with polyethylene glycol, and vacuum-dry at 50°C to obtain pre-purified ornithine aspartate;
[0063] (2) In a 2L configuration container, add 50% of the prescribed amount of water for injection, pass nitrogen to saturation, slowly add the prescribed amount of metal ion complexing agent disodium edetate, stir and dissolve;
[0064] (3) Use acetic acid-sodium acetate buffer to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com