Biology base benzoxazine containing double bond active functional groups and preparation method thereof
A technology of active functional group and benzoxazine, which is applied in the field of benzoxazine and its preparation, achieves the effects of high yield, improved heat resistance and glass transition temperature, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] At room temperature, bisphenol (E)-1,2-bis(3-methoxy-4-hydroxyphenyl)ethylene, furfurylamine and paraformaldehyde were added to a round-bottomed flask filled with solvent toluene. The molar ratio of phenol, furfurylamine and paraformaldehyde is 1:2:4.1, heat and reflux for water separation for 2 days, after the reaction is complete, extract and dry, and separate by column chromatography to obtain bio-based benzoxazine containing double bond active functional groups monomer. The reaction principle is:
[0032]
[0033] Pour the above-prepared benzoxazine monomer into a circular aluminum mold, place it in an electric heating constant temperature drying oven, and cure it in stages at different temperatures to obtain ring-opening polymerized polybenzoxazine. The heating and curing process is as follows: first Heat up to 180°C for 2 hours, then heat up to 200°C for 2 hours, and finally heat up to 220°C for 2 hours.
Embodiment 2
[0035] At room temperature, bisphenol (E)-1,2-bis(3-methoxy-4-hydroxyphenyl)ethylene, furfurylamine and paraformaldehyde were added to a round-bottomed flask filled with solvent toluene. The molar ratio of phenol, furfurylamine and paraformaldehyde is 1:2:4.4, the temperature of the oil bath is raised to 130°C, heated to reflux for water separation reaction for 3 days, after reaching the theoretical water production of 1.3ml, extract with ethyl acetate, Washing with 10% sodium hydroxide aqueous solution, distilled water and saturated saline successively, drying with anhydrous sodium sulfate, and separating by column chromatography to obtain bio-based benzoxazine monomers containing double bond active functional groups.
[0036] Pour the above-prepared benzoxazine monomer into a circular aluminum mold, place it in an electric heating constant temperature drying oven, and cure it in stages at different temperatures to obtain ring-opening polymerized polybenzoxazine. The heating a...
Embodiment 3
[0038] At room temperature, add 5g of bisphenol (E)-1,2-bis(3-methoxy-4-hydroxyphenyl)ethylene and 2.32g of paraformaldehyde into a 100mL round bottom flask filled with 60mL of toluene solvent , and then dropwise added 3.4ml furfurylamine, the temperature of the oil bath was raised to 130°C, and heated to reflux for water separation for 2 days. Washing with distilled water and saturated saline, drying with anhydrous sodium sulfate, and separating by column chromatography to obtain a bio-based benzoxazine monomer containing a double bond active functional group.
[0039]Weigh 20g of benzoxazine monomer, put it into a round aluminum mold with a diameter of 10cm, place it in an electric constant temperature drying oven, and heat it up to make it solidify. 3h, then heat curing at 200°C for 3h, and finally heat curing at 220°C for 3h to obtain ring-opening polymerized polybenzoxazine.
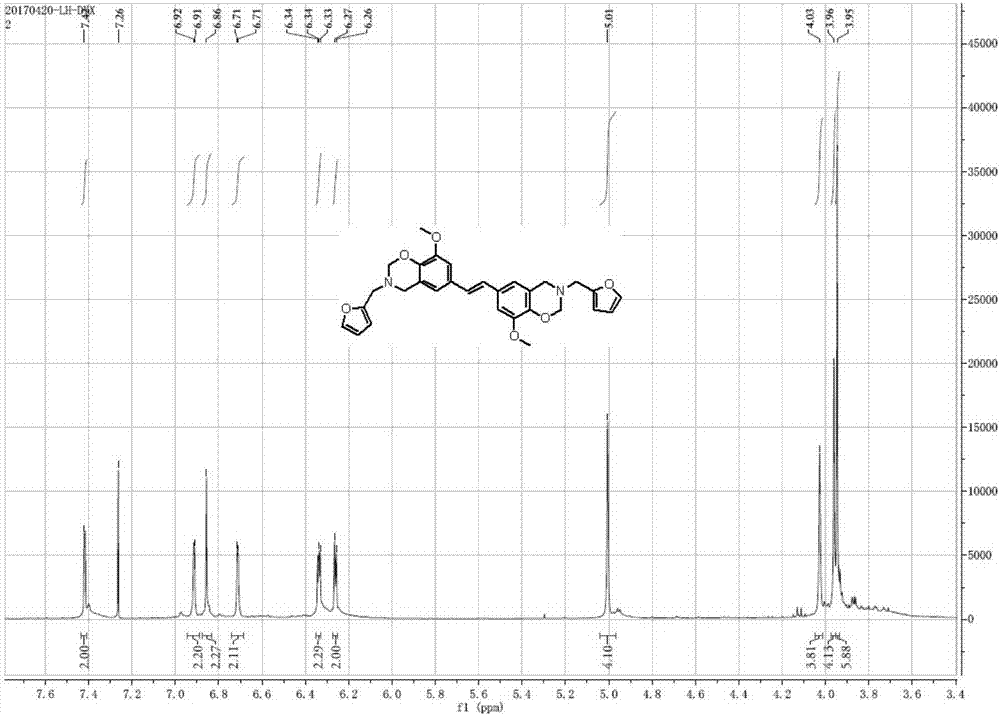
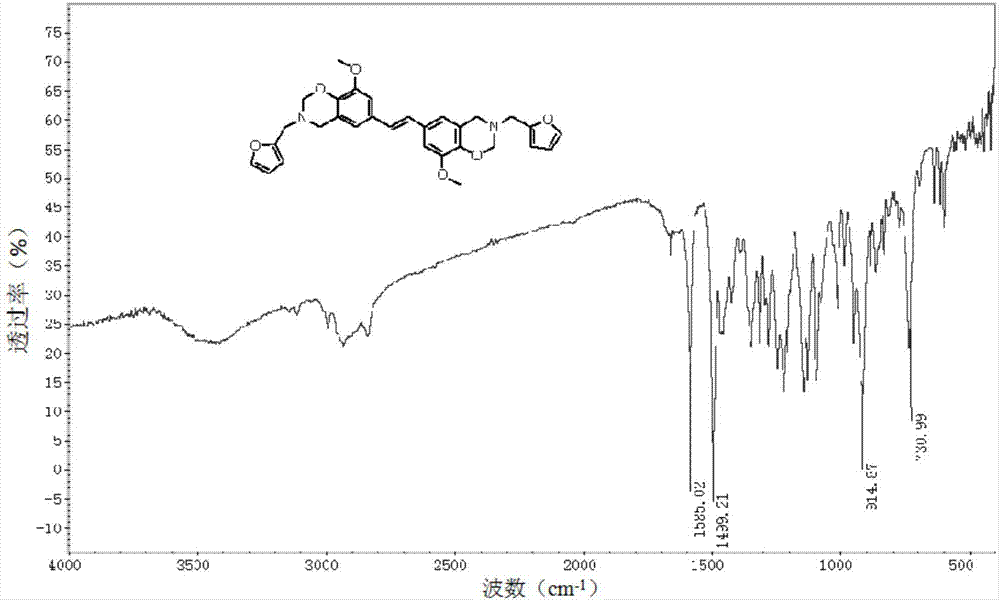
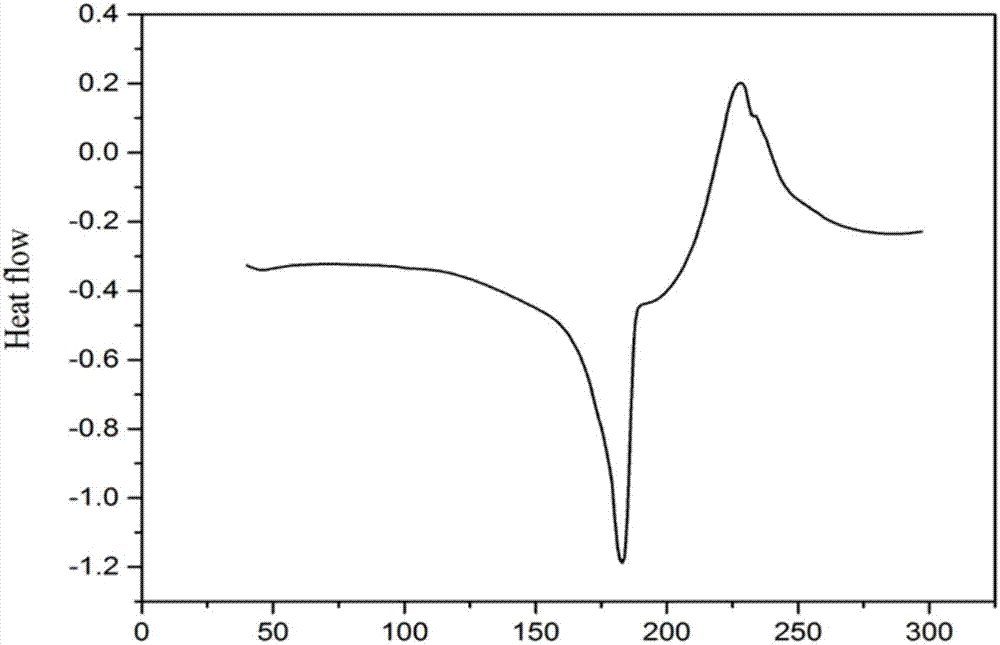
[0040] The benzoxazine monomer and polybenzoxazine prepared in Examples 1, 2 and 3 were subject...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com