Indenoimidazole compound, material comprising the indenoimidazole compound, and organic electroluminescent device
An electroluminescent device, indenimidazole technology, applied in the field of organic electroluminescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
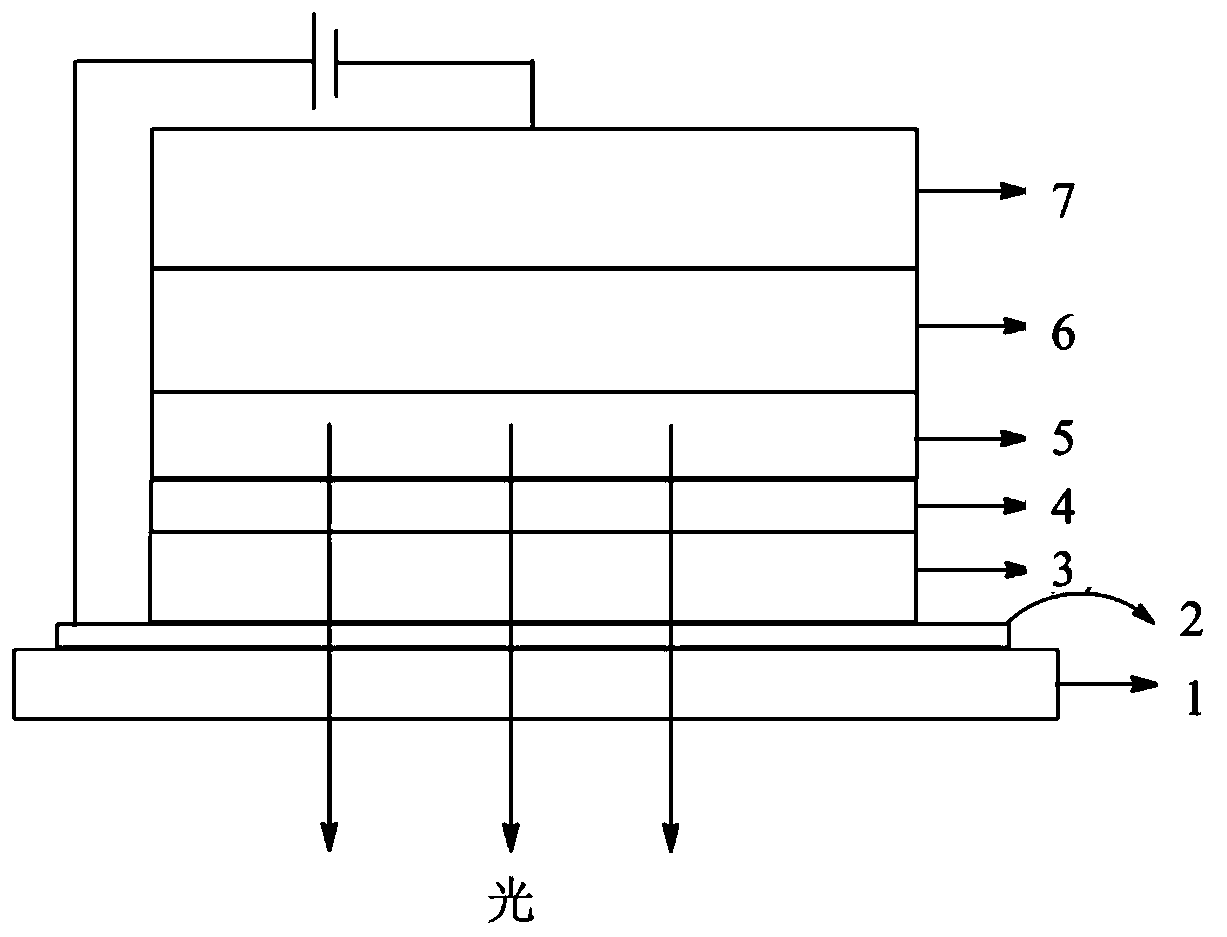
Image
Examples
Embodiment 1
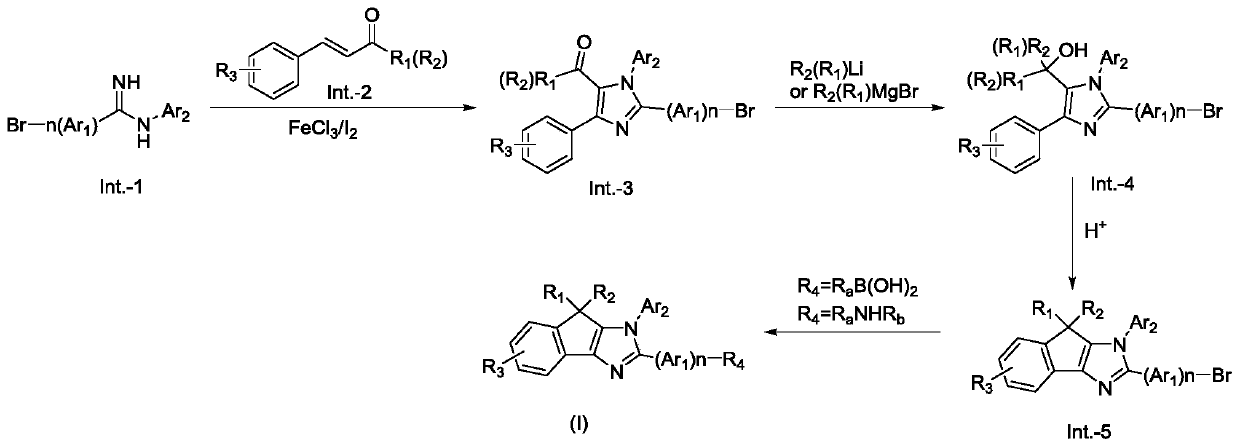
[0084] The structural formula of compound CJH-P16 is shown in the following formula:
[0085]
[0086] Its preparation route is as follows:
[0087] The first step: preparation of intermediate Int.-2
[0088]
[0089] 15g (54.5mmol) of 4-bromo-N-phenylbenzamidine Int.-1 and 9.4g (45.5mmol) of chalcone, 1.5g of anhydrous ferric chloride, then add 1.2g of iodine, add 80ml of dichlorobenzene, fed with oxygen, heated to 110°C and stirred for 8 hours, cooled to room temperature, poured the reaction solution into 800ml of petroleum ether, filtered, washed the filter cake with petroleum ether, and recrystallized with ethanol to obtain 16g of yellow solid Int.-2, yield 75%.
[0090] The second step: the preparation of intermediate Int.-3
[0091]
[0092] 15g (31.3mmol) of intermediate Int.-2 was dissolved in 150ml of anhydrous tetrahydrofuran, under the protection of nitrogen, 46.9mmol of phenylmagnesium bromide in tetrahydrofuran was added dropwise, stirred at room tempe...
Embodiment 2
[0104] The structural formula of compound CJH-P48 is shown in the following formula:
[0105]
[0106] Its preparation route is as follows:
[0107] The first step: preparation of intermediate Int.-5
[0108]
[0109] The synthetic operation refers to the second step of Example 1, and replaces the phenylmagnesium bromide in the second step of Example 1 with methylmagnesium iodide to obtain the intermediate Int.-5, a black oil, with a yield of 88%.
[0110] The second step: the preparation of intermediate Int.-6
[0111]
[0112] The synthesis operation refers to the third step of Example 1, and the intermediate Int.-3 in the third step of Example 1 is replaced by the intermediate Int.-5 to obtain the intermediate Int.-6, a black oil, and the yield is 58 %.
[0113] The third step: the preparation of product CJH-P48
[0114]
[0115] 5g (10.4mmol) of intermediate Int.-6, 4g (12.5mmol) of (4-(1-phenyl-1H-benzo[d]imidazole-2-phenyl) boronic acid, 2.2g (20.8mmol) ...
Embodiment 3
[0120] The structural formula of compound CJH-P60 is shown in the following formula:
[0121]
[0122] Its preparation route is as follows:
[0123] The first step: preparation of intermediate Int.-8
[0124]
[0125] The synthesis operation refers to the first step of Example 1, and the chalcone in the first step of Example 1 is replaced by the intermediate Int.-7 to obtain the intermediate Int.-8 as a yellow solid with a yield of 56%.
[0126] The second step: the preparation of intermediate Int.-9
[0127]
[0128] The synthetic operation refers to the second step of Example 1, replace the phenylmagnesium bromide in the second step of Example 1 with methylmagnesium iodide, and replace the intermediate Int.-2 in the second step of Example 1 with intermediate Int.-8, the intermediate Int.-9 was obtained as a black oil, and the yield was 87%.
[0129] The third step: the preparation of intermediate Int.-10
[0130]
[0131] Dissolve the intermediate Int.-9 prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com