Myricetin derivative containing amide thioether thiadiazole, and preparation method and application of same
A technology containing amide sulfides and thiadiazoles, which can be used in botany equipment and methods, chemicals for biological control, biocides, etc., and can solve problems such as instability, unfavorable absorption and release, and influence on drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
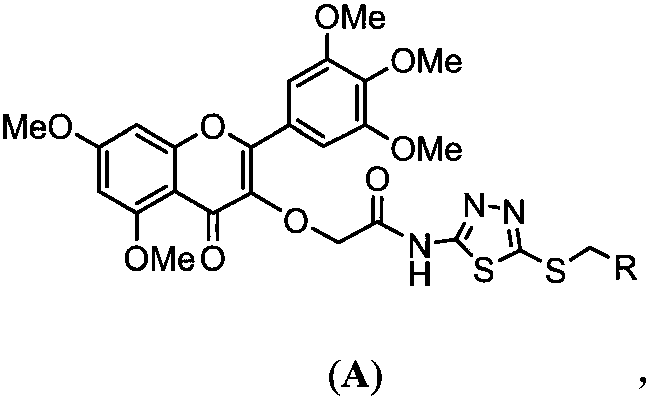
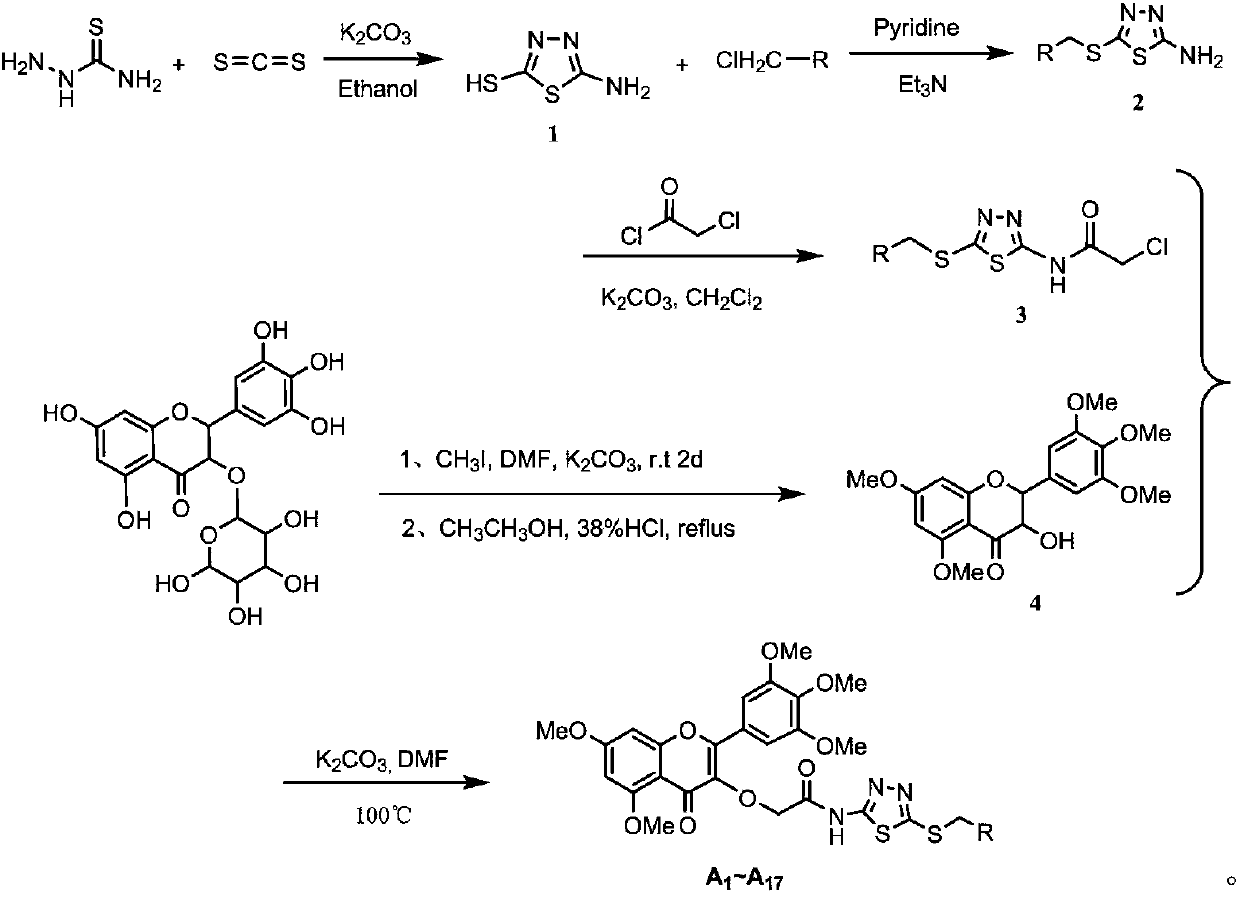
[0049] 3-O-(N-(5-((ethyl)mercapto)-1,3,4-thiadiazol-2-yl)-acetamido-2-yl)-3',4',5', 5,7-pentamethoxymyricetin (target compound A 1 ) preparation method, comprising the following steps:
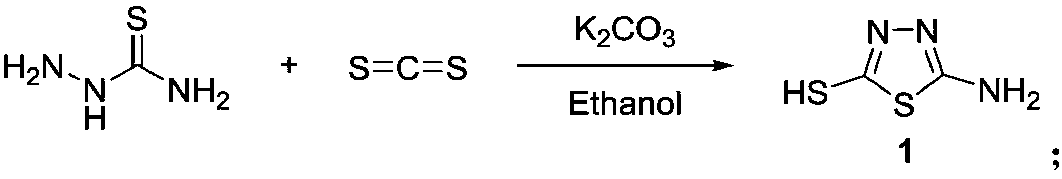
[0050] (1) Preparation of 2-amino-5-mercapto-1,3,4-thiadiazole
[0051] Add 1.03g (11.30mmol) of thiosemicarbazide and 0.78g of anhydrous (5.65mmol) potassium carbonate into a 100mL single-necked round bottom flask, add 30mL of ethanol to dissolve the thiosemicarbazide, heat and stir to reflux to boiling. 1.03g (13.56mmol) of carbon disulfide was converted into a volume of 818μL and dissolved in a 10mL measuring cup filled with 5mL of absolute ethanol, and the ethanol solution of carbon disulfide was slowly added dropwise to the reaction system, and continued to reflux for 4h. TLC traced the end of the reaction, stopped the reaction, evaporated the solvent under reduced pressure, added 30 mL of water to the residue, stirred for 10 min, acidified with 10% hydrochloric acid, filtered and washe...
Embodiment 2
[0061] 3-O-(N-(5-((phenyl)mercapto)-1,3,4-thiadiazol-2-yl)-acetamido-2-yl)-3',4',5', 5,7-pentamethoxymyricetin (target compound A 2 ) preparation method, comprising the following steps:
[0062] (1) Preparation of 2-amino-5-mercapto-1,3,4-thiadiazole:
[0063]As in the first (1) step of Example 1.
[0064] (2) Preparation of 2-amino-5-((phenyl)mercapto)-1,3,4-thiadiazole
[0065] As in the (2) step of Example 1, the difference is that benzyl chloride is used as a raw material.
[0066] (3) Preparation of N-(5-((phenyl)mercapto)-1,3,4-thiadiazol-2-yl)-2-chloro-acetamide
[0067] As in step (3) of Example 1, the difference is that 2-amino-5-((phenyl)mercapto)-1,3,4-thiadiazole is used as the raw material.
[0068] (4) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0069] As embodiment 1 (4) step.
[0070] (5) 3-O-(N-(5-((phenyl)mercapto)-1,3,4-thiadiazol-2-yl)-acetamide-2-yl)-3',4', Preparation of 5',5,7-pentamethoxymyricetin.
[0071] As in step (5) of E...
Embodiment 3
[0073] 3-O-(N-(5-((2-chlorophenyl)mercapto)-1,3,4-thiadiazol-2-yl)-acetamido-2-yl)-3',4', 5',5,7-pentamethoxymyricetin (target compound A 3 ) preparation method, comprising the following steps:
[0074] (1) Preparation of 2-amino-5-mercapto-1,3,4-thiadiazole:
[0075] As in the first (1) step of Example 1.
[0076] (2) Preparation of 2-amino-5-((2-chlorophenyl)mercapto)-1,3,4-thiadiazole
[0077] As in step (2) of Example 1, the difference is that 2-chlorobenzyl chloride is used as a raw material.
[0078] (3) Preparation of N-(5-((2-chlorophenyl)mercapto)-1,3,4-thiadiazol-2-yl)-2-chloro-acetamide
[0079] As in step (3) of Example 1, the difference is that 2-amino-5-((2-chlorophenyl)mercapto)-1,3,4-thiadiazole is used as the raw material.
[0080] (4) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0081] As embodiment 1 (4) step.
[0082] (5) 3-O-(N-(5-((2-chlorophenyl)mercapto)-1,3,4-thiadiazol-2-yl)-acetamide-2-yl)-3', Preparation of 4',5',5,7-pentame...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com