Biodegradable hydrogel as well as preparation and application thereof
A biodegradable, hydrogel technology, applied in medical science, prosthesis, etc., can solve the problem of poor acid-base biodegradability, achieve good elastic modulus and swelling performance, low cytotoxicity, and high cell affinity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) preparation of glycidyl p-aldehyde benzoate
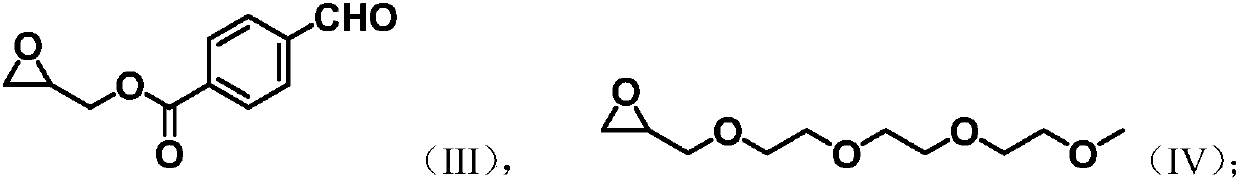
[0025] Dissolve 100mmol of 4-formylbenzoic acid, 100mmol of glycidol and 10mmol of N,N-dimethylaminopyridine (DMAP) in 500mL of dichloromethane, and stir at room temperature for 30min. At 0°C, 100 mmol of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCl) was slowly added, and then reacted overnight at room temperature. Wash with 1M hydrochloric acid, saturated aqueous sodium bicarbonate solution and saturated brine successively to remove dichloromethane to obtain the initial product. The initial product was purified by silica gel column chromatography to obtain 18 g (87 mmol) of light yellow solid.
[0026] Compound Characterization
[0027] 1 H NMR (400MHz, CDCl 3 )δ(ppm): 9.74~9.75(s,1H), 7.93~8.04(t,4H), 4.51~4.53(m,1H), 4.27~4.28(m,1H), 3.22~3.23(m,1H) ,2.61~2.63(m,1H),2.33~2.34(m,1H)
[0028] 13 C NMR (200MHz, CDCl3) δ (ppm): 189.3, 163.6, 143.8, 138.4, 131.5, 129.1, 67.8, 52.5, 44.3
[002...
Embodiment 2
[0069] (1) Preparation of aldehyde-containing aliphatic polyester
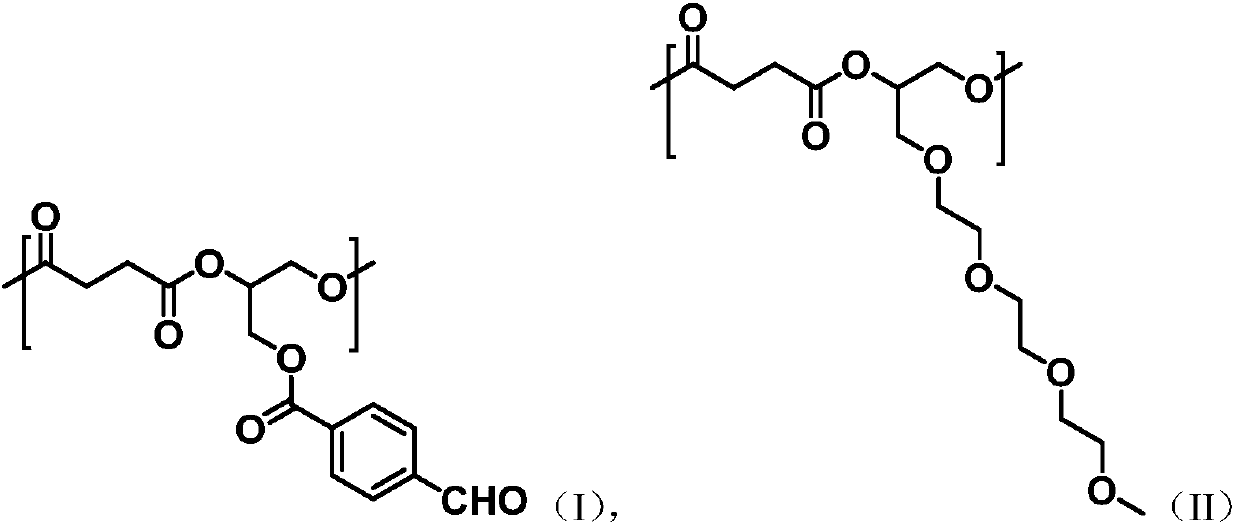
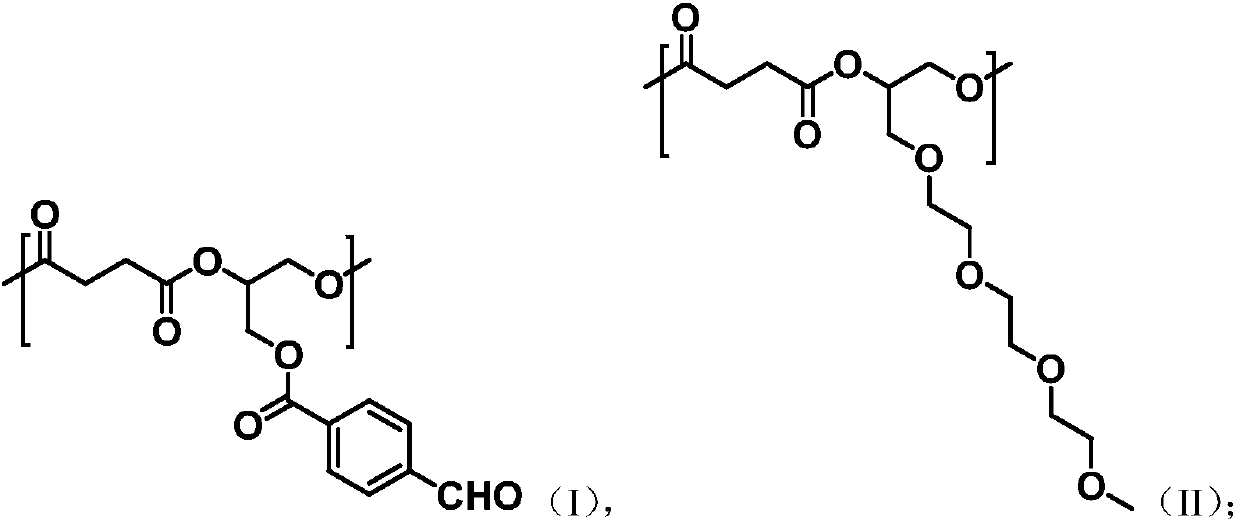
[0070] Take succinic anhydride (2g, 20mmol), glycidyl p-aldehyde benzoate (2.06g, 10mmol), triethylene glycol glycidyl ether (2.2, 10mmol), o-tert-butylhydroquinone (66.4mg , 0.4mmol) and tetrabutylammonium bromide (128mg, 0.4mmol), were added to 20mL ethyl acetate: butyl acetate = 1:1 volume ratio mixed solution, under the protection of nitrogen, 90 ℃ ring-opening copolymerization reaction 8h, remove the solvent to obtain aldehyde-containing aliphatic polyester.
[0071] Adopt the same method of embodiment 1 to detect the prepared aldehyde-containing aliphatic polyester, the result is: M w =8034, M n =3998, PDI=2.01.
[0072] (2) Preparation and properties of readily biodegradable hydrogels
[0073] (a) Formulation of readily biodegradable hydrogels
[0074] 5 g of aldehyde-containing aliphatic polyester prepared in step 1, and 0.25 g of cystamine.
[0075] (b) Preparation of readily biodegradable hydro...
Embodiment 3
[0093] (1) Preparation of aldehyde-containing aliphatic polyester
[0094] Take succinic anhydride (2g, 20mmol), glycidyl p-aldehyde benzoate (2.06g, 10mmol), triethylene glycol glycidyl ether (2.2, 10mmol), o-tert-butylhydroquinone (66.4mg , 0.4mmol) and tetrabutylammonium bromide (128mg, 0.4mmol), were added to 20mL ethyl acetate: butyl acetate = 1:1 volume ratio mixed solution, under the protection of nitrogen, 110 ℃ ring-opening copolymerization reaction 8h, remove the solvent to obtain aldehyde-containing aliphatic polyester.
[0095] Adopt the same method of embodiment 1 to detect the prepared aldehyde-containing aliphatic polyester, the result is: M w =12906, M n =6003, PDI=2.15.
[0096] (2) Preparation and properties of readily biodegradable hydrogels
[0097] (a) Formulation of readily biodegradable hydrogels
[0098] 5 g of aldehyde-containing aliphatic polyester prepared in step 1, and 1 g of cystamine.
[0099] (b) Preparation of readily biodegradable hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com