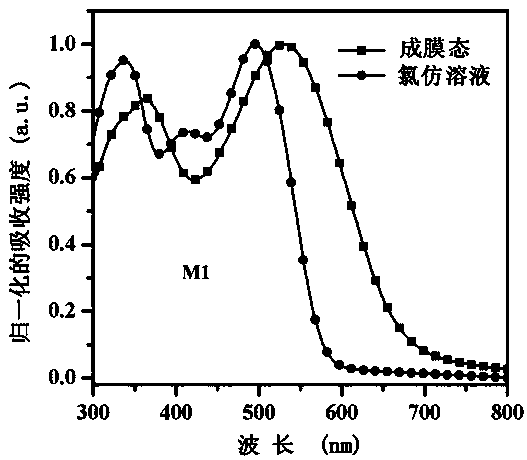
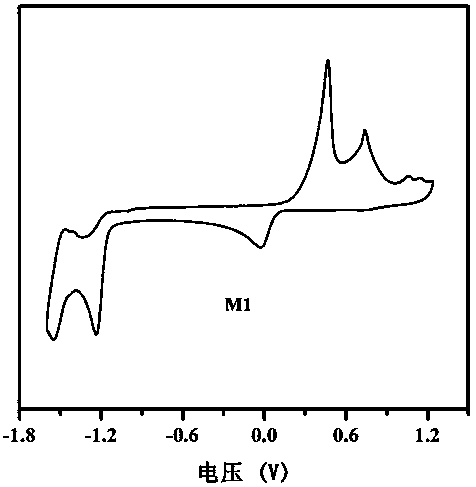
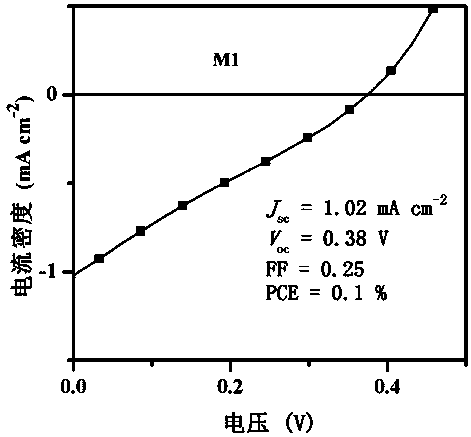
Benzothiadiazole unit-based A-pi-A'-pi-A type acceptor photovoltaic material, and preparation method and application thereof
A technology of benzothiadiazole and photovoltaic materials, which is applied in the fields of organic semiconductor device materials, photovoltaic power generation, semiconductor/solid-state device manufacturing, etc., can solve the problems of long synthesis steps and lack of systematic research on the photovoltaic performance of materials, and achieve the goal of synthesis Fewer steps, improved planarity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This example discloses the specific synthesis process of the organic small molecule donor material M1, the steps are as follows:
[0055] Under nitrogen protection, compound A' (0.25 mmol, 0.171 g), compound A1 (0.58 mmol, 0.214 g), tetrakis(triphenylphosphine) palladium (0.025 mmol, 29 mg) and potassium carbonate (10 mmol, 1.380 g ) in a 50 mL three-neck flask, 10 mL of toluene, 5 mL of ethanol, and 5 mL of water were added in sequence, and heated at reflux at 110 °C for 48 h. The reaction solution was cooled to room temperature, poured into 20 mL of water and extracted with dichloromethane (3 × 30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was extracted with petroleum ether at a volume ratio of 3:1 / dichloromethane as developing solvent for column chromatography and recrystallization with petroleum ether / ethanol to obtain 0.183 g of black powder with a yield of 71.8%...
Embodiment 2
[0062] This example discloses the specific synthesis process of the organic small molecule donor material M2, including the following steps:
[0063] Under nitrogen protection, compound A' (0.25 mmol, 0.171 g), compound A2 (0.58 mmol, 0.214 g), tetrakis(triphenylphosphine) palladium (0.025 mmol, 29 mg) and potassium carbonate (10 mmol, 1.380 g ) in a 50 mL three-neck flask, 10 mL of toluene, 5 mL of ethanol, and 5 mL of water were added in sequence, and heated at reflux at 110 °C for 48 h. The reaction solution was cooled to room temperature, poured into 20 mL of water and extracted with dichloromethane (3 × 30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The volume ratio of the crude product was 3:2 to 1: 1 petroleum ether / dichloromethane was used as developing solvent for column chromatography gradient separation and recrystallization with petroleum ether / dichloromethane / ethanol to obtain 0.23...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com