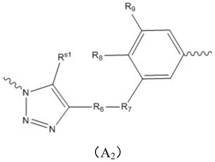
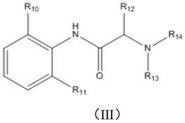
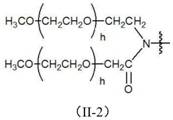
Application of a combination of polyethylene glycol and local anesthetic in non-narcotic analgesia
A technology of local anesthetic and polyethylene glycol, applied in the field of medicine, can solve the problems of short duration of non-narcotic analgesia, difficult to control, small window of high and low doses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Synthesis of embodiment 1 connecting chain (L1)
[0144]
[0145] Add 4-pentynoic acid (1.96g, 20mmol) and N,N-dicyclohexylcarbodiimide (DCC, 5.16g, 25mmol) into dichloromethane (50mL), cool in an ice-water bath, and then add p-hydroxyl Benzaldehyde (2.68g, 22mmol), the ice bath was removed after the addition, and reacted at room temperature overnight. After filtration, the filter cake was washed with ethyl acetate, and the filtrate was evaporated to dryness to obtain a crude product, which was purified by column chromatography to obtain 3.52 g of product 1a.
[0146] Compound 1a (3.23g, 16mmol) was added to anhydrous methanol (35mL), cooled to 0°C, then sodium borohydride (365mg, 9.6mmol) was added, reacted at the same temperature for 10min, and then quenched with 1M HCl, Methanol was spin-dried, ethyl acetate and saturated brine were added, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, dried, fil...
Embodiment 2
[0148] The synthesis of embodiment 2 lidocaine quaternary ammonium salts (Y1)
[0149]
[0150] Lidocaine (0.50 g, 2.13 mmol) and compound L1 (1.00 g, 3.19 mmol) were added into acetonitrile (20 mL), and reacted overnight at 50°C. TLC monitoring showed that the reaction of lidocaine was complete, and the reaction solution was concentrated to obtain a crude product. Purification by column chromatography yielded 1.19 g of product Y1. 1 H NMR: (CDCl 3 ): 1.59(m, 6H), 2.06(s, 2H), 2.31(s, 6H), 2.64(m, 2H), 2.87(m, 2H), 3.49(m, 2H), 3.71(m, 2H) , 4.89 (s, 2H), 4.93 (s, 2H), 7.06 (m, 3H), 7.28 (d, 2H), 7.62 (d, 2H), 9.79 (s, 1H).
Embodiment 3
[0151] Synthesis of Example 3 lidocaine conjugate 1 (mPEG-lidocaine, 20K)
[0152]
[0153] mPEG-N 3 (20K, 2.00g, 0.10mmol), compound Y1 (65.8mg, 0.12mmol), vitamin C (52.8mg, 0.30mmol) were added to N,N-dimethylformamide (20mL), stirred rapidly to dissolve , and then copper sulfate pentahydrate (30.0 mg, 0.12 mmol) in water (4.4 mL, 2.2 mL / g PEG) was added, reacted overnight at room temperature, and precipitated with isopropanol to obtain 1.90 g of product. 1 H NMR: (CDCl 3 ): 1.42(m, 6H), 2.19(s, 6H), 3.02(m, 4H), 3.23(m, 4H), 3.31(s, 3H), 3.50(m, 1800H), 3.80(m, 2H) , 4.20(m, 2H), 4.50(s, 2H), 4.82(s, 2H), 7.12(m, 3H), 7.30(d, 2H), 7.64(d, 2H), 7.91(s, 1H), 10.28(s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com