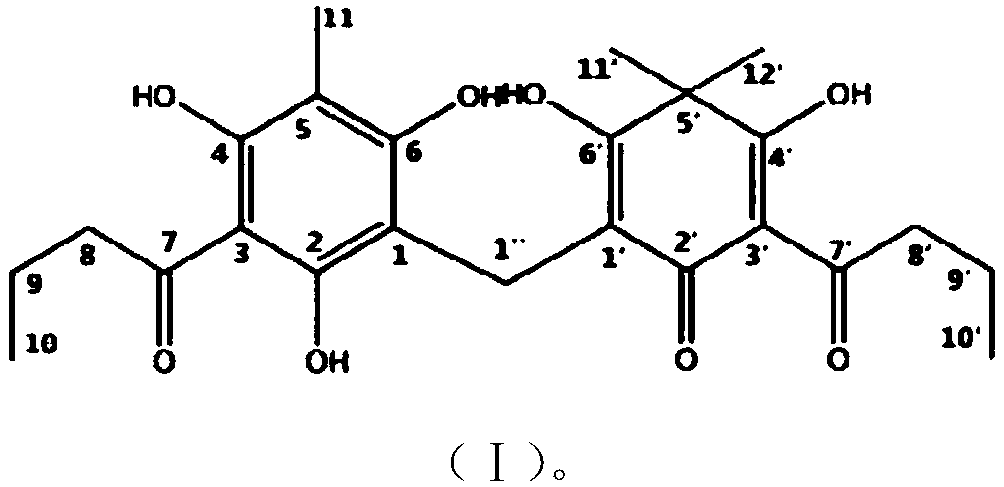
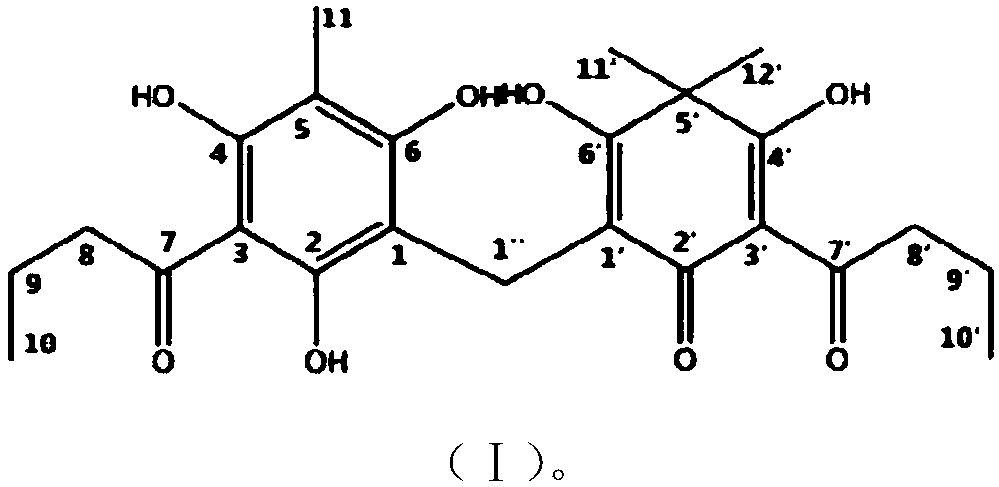
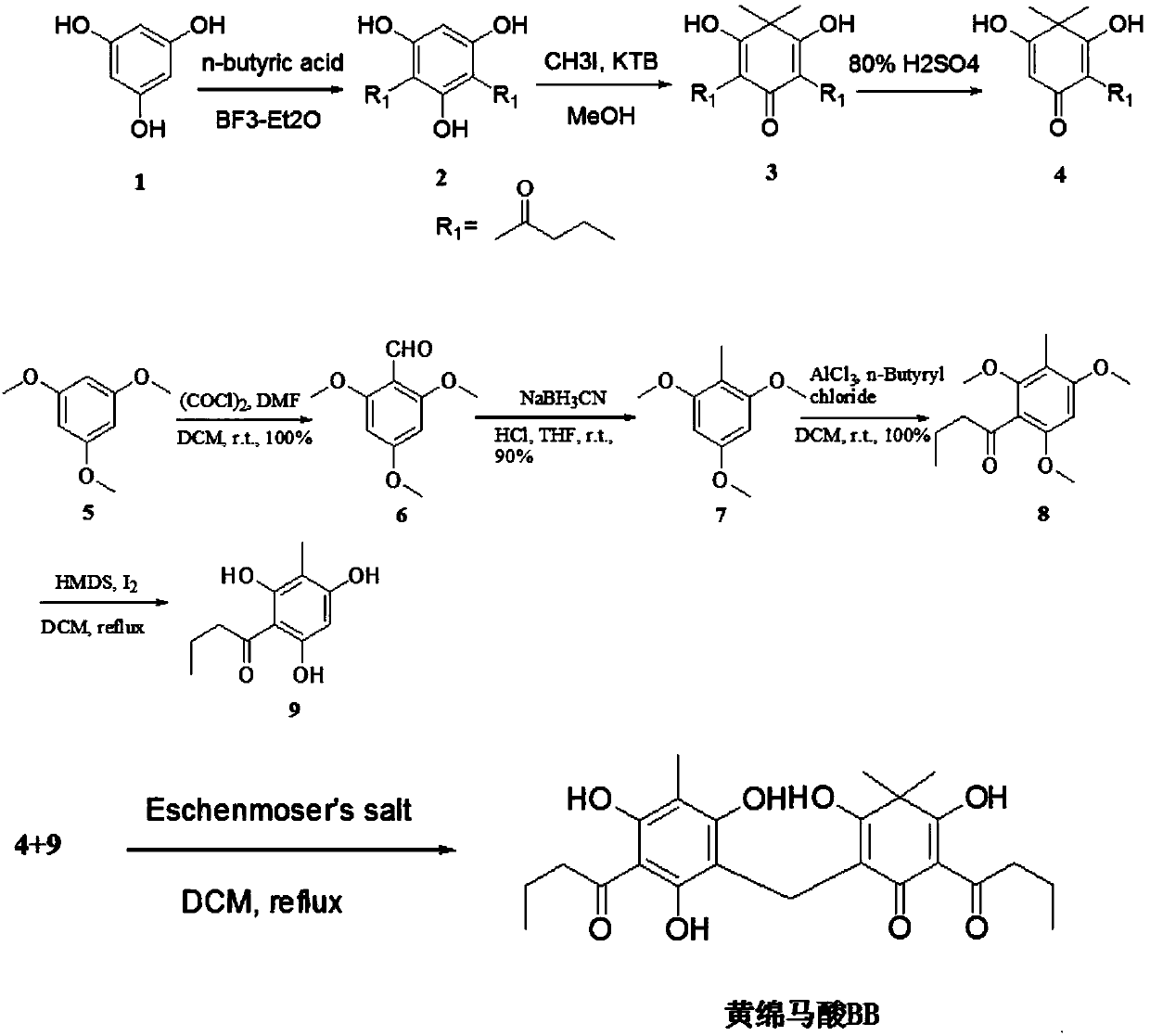
Dryopteris fragrans phloroglucinol compound flavaspidic acid BB and antibacterial application thereof
A technology of phloroglucinol and fumaric acid, which is applied in the fields of chemistry and medicine, and can solve the problems of anti-bacteria that have not been reported in technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 The separation and preparation of phloroglucinol compound fumaric acid BB
[0047]S1. Extraction of total phloroglucinol compounds of Trichophyllum chinensis: extract 10 kg dry coarse powder of Trichomona citrifolia by cold soaking with 10 times the volume of 50% edible ethanol for 2 times, each time for 24 hours, and concentrate under reduced pressure to recover ethanol To obtain the sample aqueous solution without alcohol smell;
[0048] S2. Adjust the pH to 1.5-4.5 with hydrochloric acid, let stand for 12-18 hours, and centrifuge to obtain 1.2 kg of precipitated extract.
[0049] S3. After dissolving the precipitated extract sample obtained in S2, mix the sample with silica gel, perform preliminary separation on a silica gel chromatographic column, and use different proportions of petroleum ether-acetone (100:1~1:1) successively to obtain the corresponding component Fr- A~E.
[0050] The silica gel chromatographic column uses silica gel (60-100 mesh) to m...
Embodiment 2
[0054] The chemical synthesis of embodiment 2 phloroglucinol compounds fumaric acid BB
[0055] At present, the way to obtain the compound fumaric acid BB is mainly to separate and purify from plants, and the separation and preparation are cumbersome and the yield is low. This example proposes for the first time the chemical synthesis pathway and design of pentamaic acid BB, which can obtain the compound in large quantities and quickly. In this example, phloroglucinol (compound 1) was used as the starting material for the reaction, and intermediate 4 was obtained through fluorine-gram acylation reaction, methylation of methyl iodide, and monodebutyrylation.
[0056] The environmentally friendly reagent oxalyl chloride was reacted with DMF to generate the vilsmeier reagent, and then reacted with the raw material 1,3,5-trimethoxybenzene (5), using sodium cyanoborohydride as the reducing agent, and the compound 7 was obtained in a yield of 90%. , and then by Friedel-Crafts acyla...
Embodiment 3
[0076] Example 3 In vitro anti-superficial fungus effect experiment of Phloroglucinol compound of Phloroglucinol
[0077] (1) Microsporum canis (CMCC(F)T 5C ), Malassezia furfur (CMCC(F)T 17a ), Microsporum gypsumus (CMCC(F) M2C ), provided by the Institute of Dermatology, Chinese Academy of Medical Sciences (Nanjing). The microdilution method of dermatophyte is carried out according to the M38-A2 scheme formulated by CLSI in the United States, which is briefly described as follows: Scrape the colony on the surface of the SDA medium with an inoculation loop, grind the mycelia with a sterile grinder, and suspend the dermatophyte in In sterile normal saline, adjust the turbidity to 0.5 McFarland turbidity, and count the number of spores and short hyphae with a hemocytometer. Make the bacterial concentration 1×10 3 CFU / mL to 3×10 3 CFU / mL. That is, the 0.5 McFarland turbidity bacteria solution was diluted 1000 times with RPMI-1640 liquid medium, and counted by the hemocytom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com