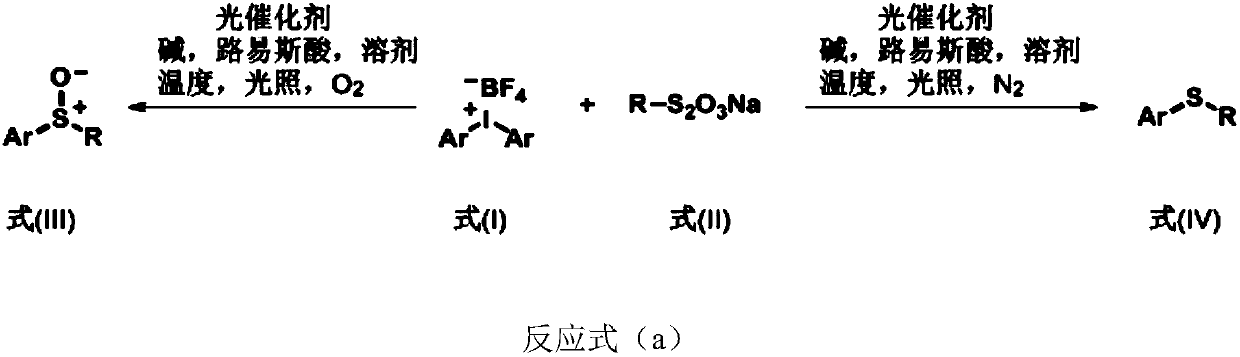
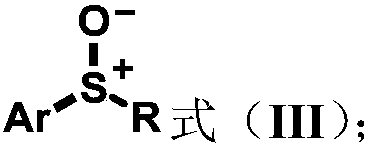Aryl sulfoxide, thioether compound, synthesis method and application thereof
A technology of thioether compounds and synthesis methods, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, and compounds of Group 5/15 elements of the periodic table, etc., which can solve the problem of danger, heavy taste of raw materials, and non-compliance with the development trend of green chemistry And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of Phenyl(n-Pentyl)sulfoxide 3a
[0064]
[0065] In a 25mL reaction tube, add Eosin Y (2.5mg, 4*10 -3 mmol), substrate 1a (73.6mg, 0.2mmol), substrate 2a (123.6mg, 0.6mmol), zinc acetate [Zn(OAc) 2 ] (73.4mg, 0.4mmol), diisopropylethylamine (DIPEA) (66μL, 0.4mmol), methanol / acetonitrile (MeOH / MeCN) (0.3 / 0.1mL), stirred under 18-watt green LED light After 12 hours, the air inside the reaction tube was purged and replaced, and the reaction was continued for 12 hours after sealing. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography (PE / EA=2 / 1) to obtain a colorless liquid 3a (32.2mg, 82%), R f =0.5 (PE / EA=2 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.60 (dt, J=8.5, 2.2Hz, 2H), 7.55–5.14 (m, 3H), 2.76 (ddd, J=8.5, 6.6, 1.8Hz, 2H), 1.72 (ddd, J=8.7, 7.2,3.0Hz,1H),1.61(dt,J=20.2,7.5Hz,1H),1.47–1.25(m,4H),0.86(t,J=7.1Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ140.0, 130.8, 129.1, 124.0, 57.3, 30.7, 22.2, 21.8, 13.7, IR (KBr...
Embodiment 2
[0067] Synthesis of 4-tert-butylphenyl(n-pentyl)sulfoxide 3b
[0068]
[0069] In a 25mL reaction tube, add Eosin Y (2.5mg, 4*10 -3 mmol), substrate 1b (108.5mg, 0.2mmol), substrate 2a (123.6mg, 0.6mmol), zinc acetate [Zn(OAc) 2] (73.4mg, 0.4mmol), diisopropylethylamine (DIPEA) (66μL, 0.4mmol), methanol / acetonitrile (MeOH / MeCN) (0.3 / 0.1mL), stirred under 18-watt green LED light After 12 hours, the air inside the reaction tube was purged and replaced, and the reaction was continued for 12 hours after sealing. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography (PE / EA=2 / 1) to obtain a colorless liquid 3b (44.4mg, 88%), R f =0.5 (PE / EA=2 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.58–7.49(m,4H),2.88–2.69(m,2H),1.85–1.53(m,4H),1.50–1.23(m,15H),0.88(t,J=7.1Hz,3H) . 13 C NMR (125MHz, CDCl 3 )δ154.5, 140.7, 126.2, 123.9, 57.3, 35.0, 31.2, 30.8, 22.3, 22.0, 13.80. -1 .HRMS(EI) Calcd for C 15 h 24 OS 252.1548, Found 252.1545.
Embodiment 3
[0071] Synthesis of 4-trifluoromethylphenyl(n-pentyl)sulfoxide 3c
[0072]
[0073] In a 25mL reaction tube, add Eosin Y (2.5mg, 4*10 -3 mmol), substrate 1c (141.1mg, 0.2mmol), substrate 2a (123.6mg, 0.6mmol), zinc acetate [Zn(OAc) 2 ] (73.4mg, 0.4mmol), diisopropylethylamine (DIPEA) (66μL, 0.4mmol), methanol / acetonitrile (MeOH / MeCN) (0.3 / 0.1mL), stirred under 18-watt green LED light After 12 hours, the air inside the reaction tube was purged and replaced, and the reaction was continued for 12 hours after sealing. After the reaction was completed, it was filtered, concentrated, and separated by column chromatography (PE / EA=2 / 1) to obtain a colorless liquid 3c (35.4mg, 67%), R f =0.5 (PE / EA=2 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.75(q,J=8.4Hz,4H),2.90–2.66(m,2H),1.88–1.69(m,1H),1.70–1.52(m,1H),1.52–1.26(m,4H) ,0.95–0.79(m,3H). 19 F NMR (376MHz, CDCl 3 )δ-62.84. 13 C NMR (100MHz, CDCl 3 )δ148.4, 133.0 (q, J = 32.8Hz), 126.2 (q, J = 3.7Hz), 127.6–118.7 (m, J = 290.9Hz), 124...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com