Synthetic method for p-hydroxybenzaldehyde
A technique for the synthesis of p-hydroxybenzaldehyde and its synthesis method, which is applied in the field of synthesis of p-hydroxybenzaldehyde, can solve problems such as high cost, shortage of raw materials, complex catalyst preparation process, etc., achieve short reaction time, increase yield and purity, and solve cost higher effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
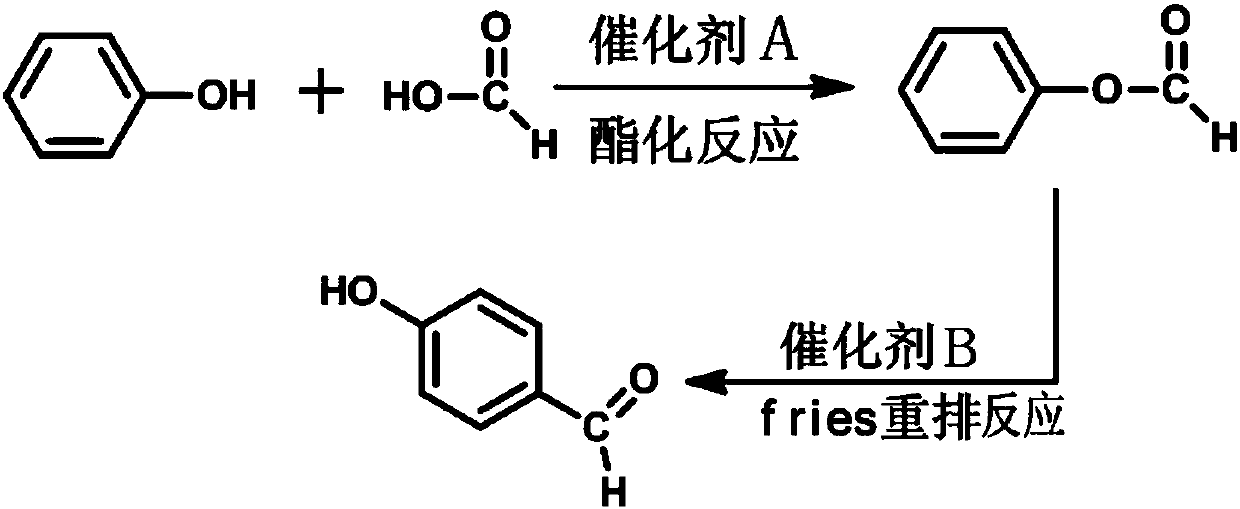
[0025] Such as figure 1 As shown, the method of the present embodiment includes the following steps:
[0026] Step 1. Weigh 18.8g (0.2mol) of phenol, 11.04g (0.24mol) of formic acid, 3.8g (0.02mol) of p-toluenesulfonic acid and 200mL of toluene, and add them to a reaction flask equipped with a heating mantle and a condensing reflux device. Under the action of magnetic stirring, heat the reaction liquid in the reaction bottle to 105°C through the heating mantle to carry out the esterification reaction for 4 hours, stop heating after the esterification reaction, and distill the reaction liquid when the temperature of the reaction liquid drops to 40°C , collecting fractions at 171°C to 173°C to obtain 24.0g of colorless phenyl formate with a yield of 98.4% and a purity of 99.2% (GC purity);
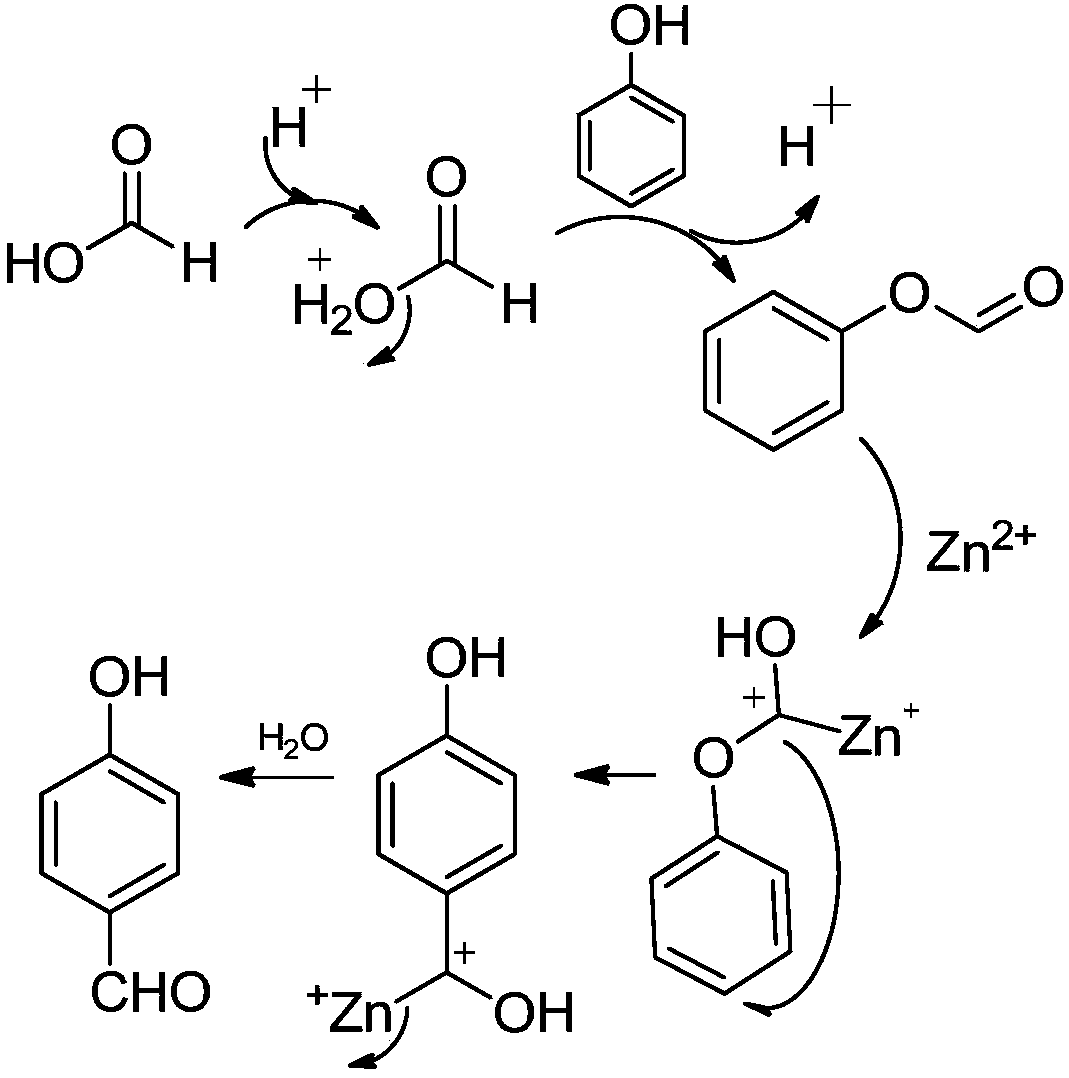
[0027] Step 2. Weigh 12.2g (0.1mol) of phenyl formate, 27.2g (0.2mol) of zinc chloride and 100mL of toluene, and add them to a reaction flask equipped with a heating mantle and a reflux con...
Embodiment 2
[0031] Such as figure 1 As shown, the method of the present embodiment includes the following steps:
[0032] Step 1. Weigh 18.8g (0.2mol) of phenol, 11.04g (0.24mol) of formic acid, 2.0g (0.02mol, 98%) of concentrated sulfuric acid and 200mL of toluene, and add them to the reaction flask equipped with a heating mantle and a condensing reflux device , under magnetic stirring, heated to 105°C; kept for 4h, stopped heating, and cooled the system; when the system temperature dropped to 40°C, distilled the system, collected fractions at 171-173°C, and obtained 23.0 g of colorless phenyl formate, Yield 94.3%, purity 99.1% (GC purity);
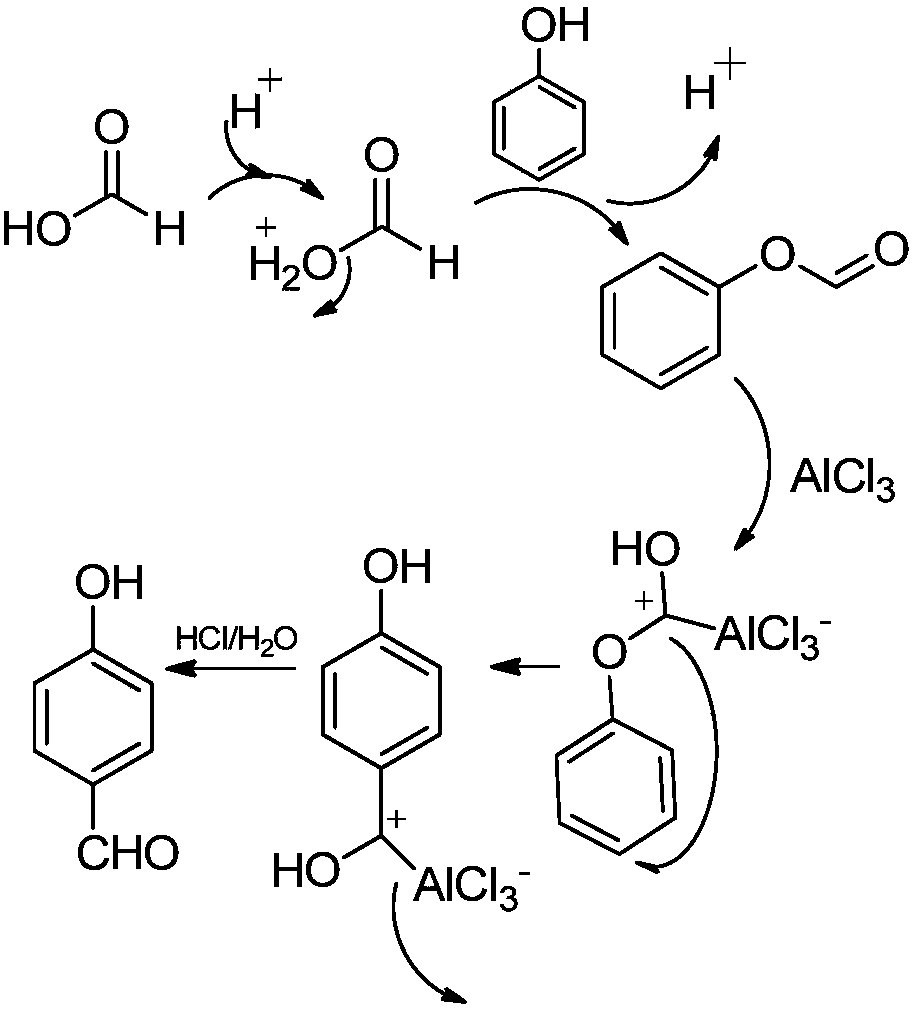
[0033] Step 2. Weigh 12.2g (0.1mol) of phenyl formate, 26.7g (0.2mol) of zinc ions or aluminum trichloride and 100mL of toluene, and add them to the reaction flask equipped with a heating mantle and a condensing reflux device, and stir under magnetic force. , heated to 110°C; after keeping for 4 hours, stop heating and cool down the system; when t...
Embodiment 3
[0037] Such as figure 1 As shown, the method of the present embodiment includes the following steps:
[0038] Step 1. Weigh 18.8g (0.2mol) of phenol, 11.04g (0.24mol) of formic acid, 3.8g (0.02mol) of p-toluenesulfonic acid and 200mL of o-dichlorobenzene, and add them to the reaction chamber equipped with heating mantle and condensing reflux In the bottle, under magnetic stirring, heat up to 180°C; after keeping for 4 hours, stop heating to cool down the system; when the system temperature drops to 40°C, distill the system, collect fractions at 171-173°C, and obtain 24.0 g of colorless phenyl formate Liquid, yield 98.4%.
[0039] Step 2. Weigh 12.2g (0.1mol) of phenyl formate, 27.2g (0.2mol) of zinc chloride and 100mL of p-xylene, and add them to the reaction flask equipped with a heating mantle and a condensing reflux device. Under magnetic stirring, heat to 135°C; after keeping for 4 hours, stop heating and cool down the system; when the temperature of the system drops to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com