Ion conductor layer modified manganese-based oxide positive electrode material, preparation method and applications thereof
A manganese-based oxide, ion conductor layer technology, applied in the field of energy storage, can solve the problems of unfavorable electrode material rate performance, extremely poor electronic conductivity, improvement and other problems, and achieve fast transmission, high energy density, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) 1g LiNi 0.5 mn 1.5 o 4 (Synthesis by solid-phase method) The material was dispersed in 80mL of ethanol, ultrasonically dispersed for 0.5h, made into suspension A, and placed in a 60°C water bath;
[0049] (2) Take 100mL 6.2*10 -3 mol / L ethanol dispersed SiO 2 Add the sol into the suspension A, then add 10mL 0.137mol / L citric acid aqueous solution and 10mL 0.1mol / L lithium hydroxide aqueous solution dropwise at 0.02mL / min and stir vigorously at a stirring speed of 800rpm. During the dropping process, use 2.5wt .% ammonia solution to adjust the pH of the reaction system to 8.5, and stir for 4 hours;
[0050] (3) Stir and evaporate to dryness at 80°C, and roast at 500°C for 5h to obtain LiNi 0.5 mn 1.5 o 4 @3wt.%Li 4 SiO 4
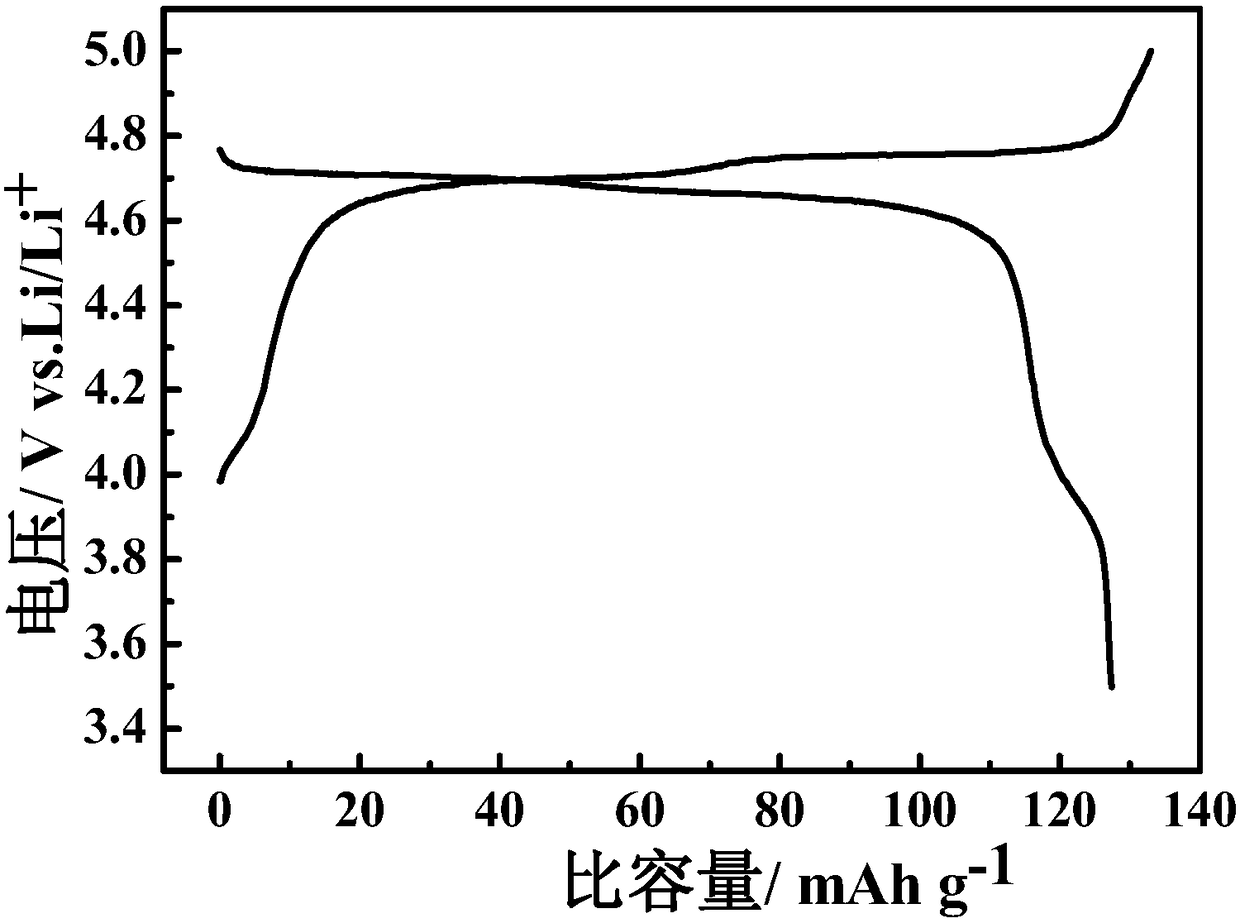
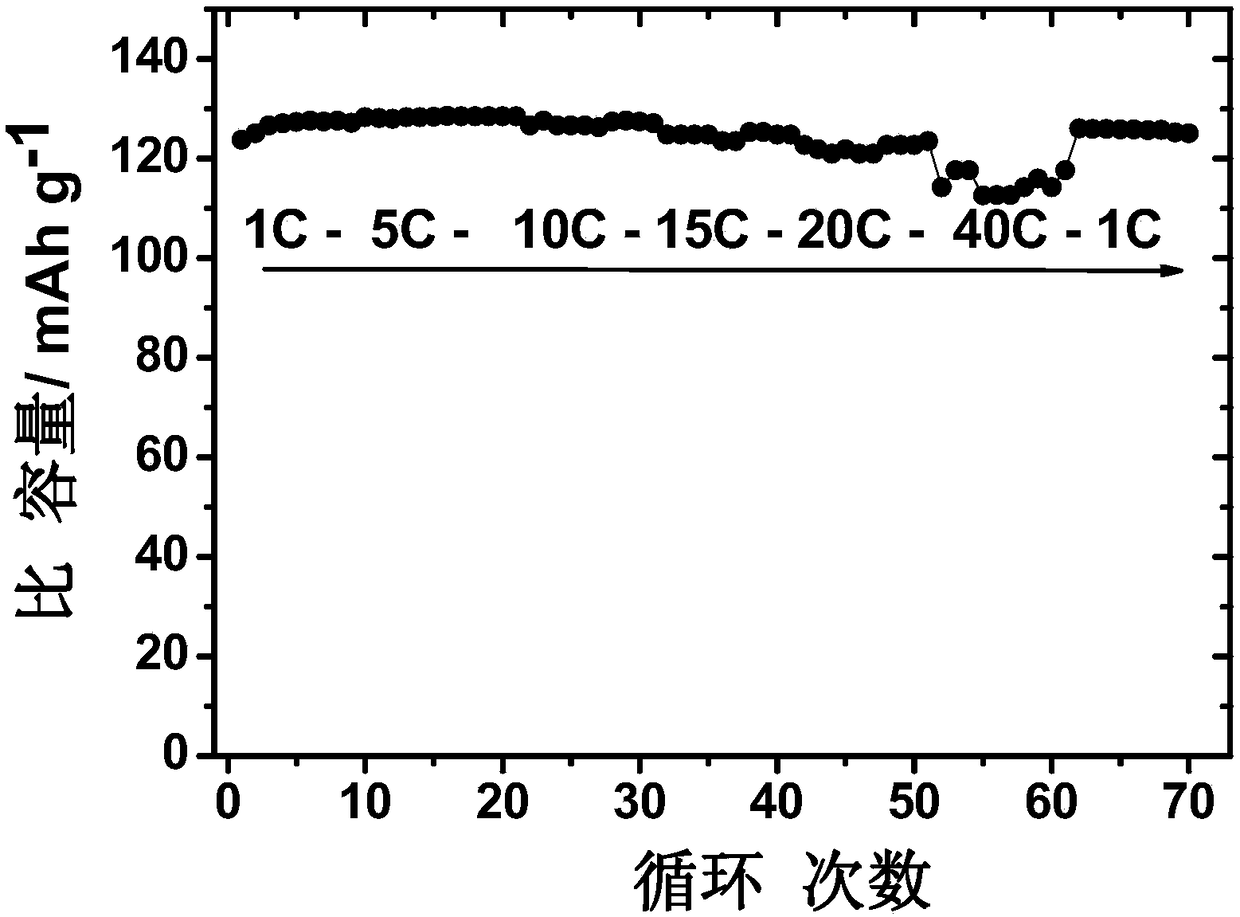
[0051] (4) Phase analysis: X-ray diffraction spectrum analysis was performed on the prepared material, which showed that the obtained material had a spinel structure, belonged to the Fd-3m crystal form, and had a high degree of crystallin...
Embodiment 2
[0056] (1) 1g Ni 0.5 mn 1.5 o x (Preparation by solid-phase method) The material is dispersed in 100mL alcohol-water mixed solution (the volume ratio of ethanol to water is 7:1), and ultrasonically dispersed into suspension A;
[0057] (2) Add 20mL of 2.5mol / L sodium silicate solution dropwise to A at 85°C at 10mL / min, adjust the pH of the reaction system to 10 with 2.5wt.% ammonia solution, and stir for 3h;
[0058] (3) The product is centrifuged, washed, dried overnight, then mixed with an excess of 10% lithium acetate, and calcined. The calcining program is 500°C for 5h+900°C for 12h+700°C for 12h.
[0059] (4) Phase analysis and morphology characterization: attached Figure 5 The X-ray diffraction spectrum of the obtained material has a spinel structure, belongs to the Fd-3m crystal form, and contains a small amount of impurity Li x Ni 1-x O(x~0.2). The microscopic morphology of the material was investigated (attached Figure 6 ), the particle size of the material i...
Embodiment 3
[0063] (1) 0.5g LiNi synthesized by liquid phase co-precipitation 0.4 Cr 0.2 mn 1.4 o 4 The material was ultrasonically dispersed in 40mL of deionized water, and 20mL of an aqueous solution containing 0.05g of polyetherimide was added and stirred for 2h, called suspension A;
[0064] (2) Add an appropriate amount of lithium acetate solid particles and stir to dissolve, add 0.03mol / L tetraethyl silicate and aluminum nitrate solution dropwise to A at 20mL / min at 80°C, and use hydrochloric acid and ammonia solution to control the reaction system Keep the pH~4.0 for 2h, and the stirring speed is 2000rpm;
[0065] (3) After the reaction solution was stirred and evaporated to dryness, it was heat treated at 500°C for 3 hours to obtain 0.88LiNi 0.4 Cr 0.2 mn 1.4 o 4 0.07 Li 4 SiO 4 0.05 Li 5 AlO 4 Material.
[0066] (4) Phase analysis and morphology characterization: X-ray diffraction spectrum shows that the obtained material has a spinel structure, which belongs to the F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com