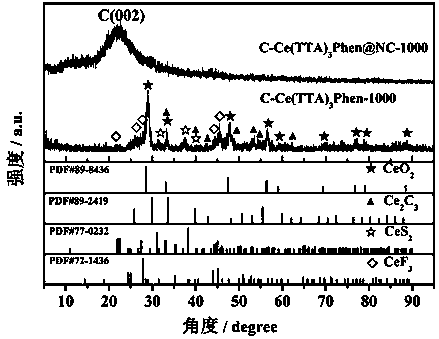
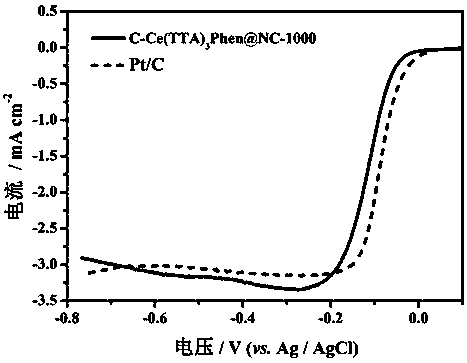
Method for preparing conductive polymer-rare-earth complex composite electric catalyst
A rare earth complex, conductive polymer technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as low initial potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Rare earth complex Ce(TTA) 3 Preparation of Phen: Dissolve 0.4976 g 2-thienoyltrifluoroacetone (TTA) and 0.1481 g o-phenanthroline (Phen) in 20 mL of absolute ethanol, adjust the pH of the solution to 7, and dissolve the above solution under constant stirring Slowly add in cerium nitrate hexahydrate (CeN 3 o 9 ·6H 2 O) in ethanol solution (0.3242 g CeN 3 o 9 ·6H 2 O dissolved in 10 mL of absolute ethanol), when precipitation appeared, continue to react at 60°C for 5 hours, after standing still for 20 hours, centrifuge the precipitate, wash with ethanol and distilled water three times respectively, and place in a constant temperature blast drying oven Drying for 10 hours gives Ce(TTA) 3 Phen complexes.
[0038] (2) Preparation of polyaniline-rare earth complex composite electrocatalyst: take the rare earth complex Ce(TTA) prepared in step (1) 3 Phen 0.50 g and sodium dodecylbenzenesulfonate (C 18 h 29 NaO 3 S, 0.59 g), was added into 80 mL ultrapure water ...
Embodiment 2
[0041] (1) Rare earth complex La(TTA) 3 The preparation of Phen: 0.4976 g 2-thienoyltrifluoroacetone (TTA) and 0.1481 g o-phenanthroline (Phen) were dissolved in 20 mL of absolute ethanol, and the pH of the solution was adjusted to 7, and the above solution was dissolved under constant stirring. Slowly add in lanthanum nitrate hexahydrate (LaN 3 o 9 ·6H 2 O) in ethanol solution (0.3234 g LaN 3 o 9 ·6H 2 O dissolved in 10 mL of absolute ethanol), when precipitation appeared, continue to react at 60 °C for 5 hours, after standing still for 20 hours, centrifuge the precipitate, wash with ethanol and distilled water three times respectively, and place in a constant temperature blast drying oven After drying for 10 hours, La(TTA) was obtained 3 Phen complexes.
[0042] (2) Preparation of polyaniline-rare earth complex composite electrocatalyst: take the rare earth complex La(TTA) prepared in step (1) 3 Phen 1.00 g and sodium dodecylbenzenesulfonate (C 18 h 29 NaO 3 S, 0....
Embodiment 3
[0045] (1) Rare earth complex Pr(TTA) 3 The preparation of Phen: 0.4976 g 2-thienoyltrifluoroacetone (TTA) and 0.1481 g o-phenanthroline (Phen) were dissolved in 20 mL of absolute ethanol, and the pH of the solution was adjusted to 7, and the above solution was dissolved under constant stirring. Slowly drop in praseodymium chloride (PrCl 3 ) in ethanol solution (0.3241 g PrCl 3 dissolved in 10 mL of absolute ethanol), when precipitation occurs, continue to react at 60 °C for 5 hours, after standing still for 20 hours, centrifuge the precipitate, wash with ethanol and distilled water for 3 times, and dry in a constant temperature blast drying oven 10 hours, get Pr(TTA) 3 Phen complexes.
[0046] (2) Preparation of polyaniline-rare earth complex composite electrocatalyst: take the rare earth complex Pr(TTA) prepared in step (1) 3 Phen 1.50 g and sodium dodecylbenzenesulfonate (C 18 h 29 NaO 3 S, 0.59 g), was added into 80 mL ultrapure water for ultrasonic dispersion for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com