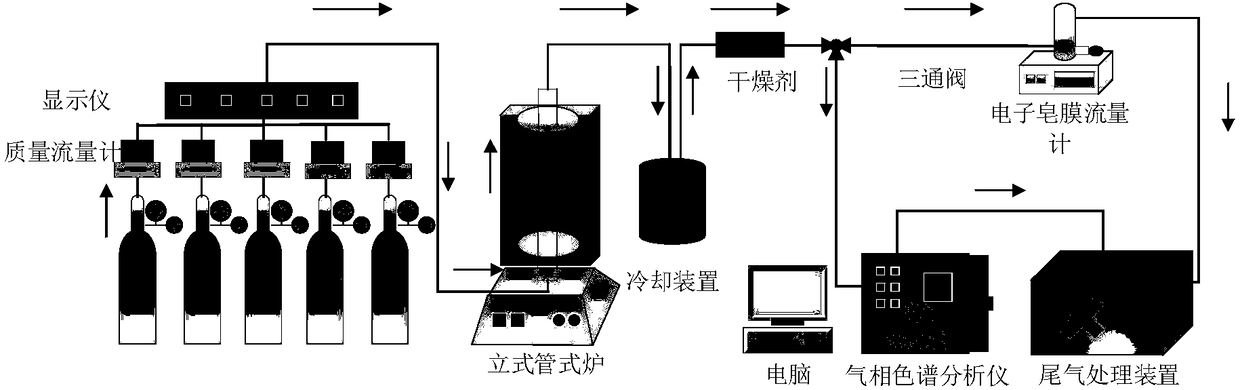
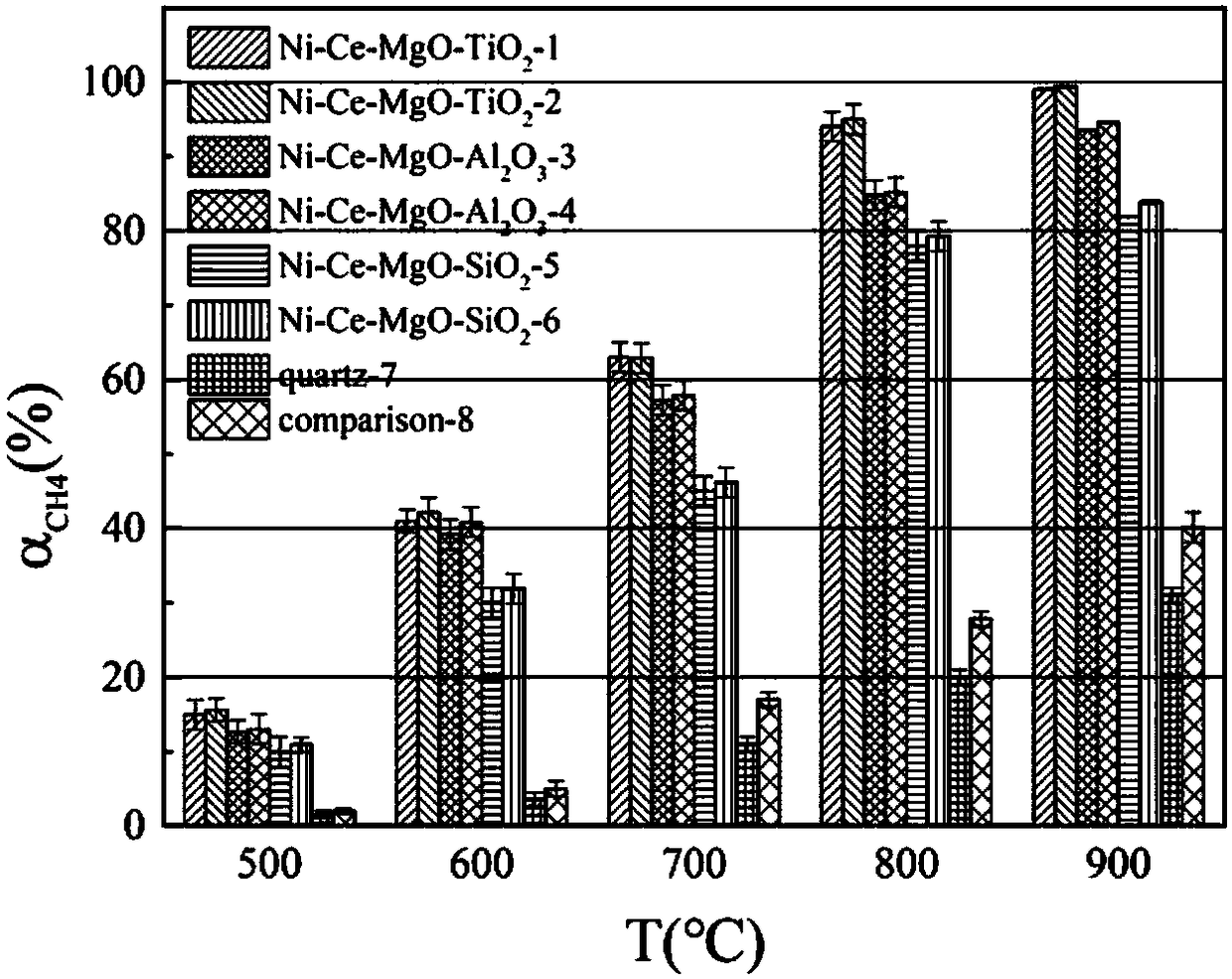
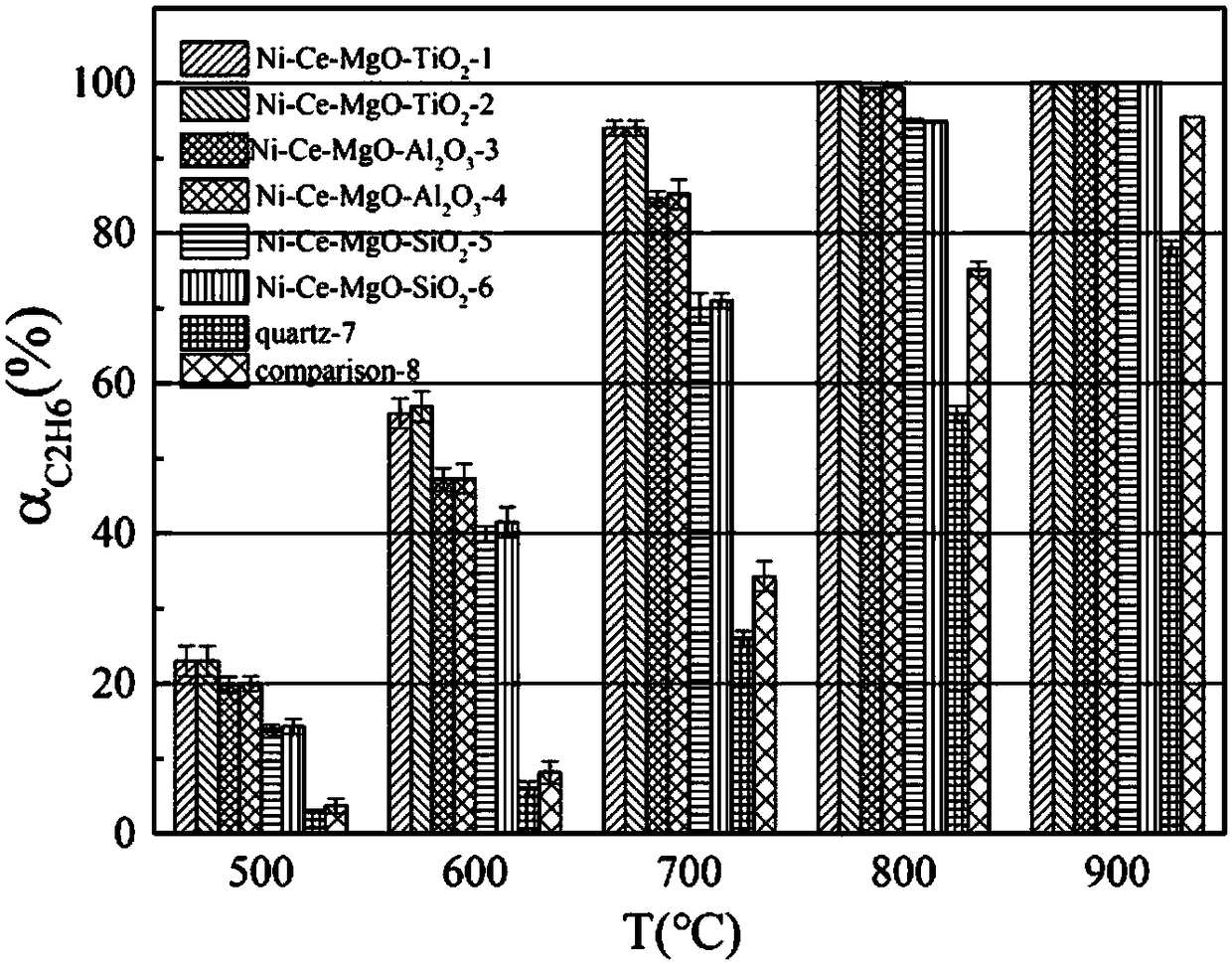
Catalyst applicable to synthesis-gas preparation by catalytic reforming of shale gas and carbon dioxide and preparation method thereof
A catalytic reforming and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., to improve adsorption, accelerate dissociation and conversion, and improve catalytic active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Measure 140mL of absolute ethanol and pour it into a beaker, measure 30.7mL of butyl titanate and slowly pour it into absolute ethanol, and continue stirring at a medium speed on a magnetic stirrer; during the above stirring process, measure 3mL of ice Add acetic acid drop by drop, and continue to stir to form a transparent solution A; weigh 2.5g of nickel nitrate hexahydrate, 1.6g of cerium nitrate hexahydrate, and 12.8g of magnesium nitrate hexahydrate, mix and dissolve in 20mL deionized water, and place on a magnetic stirrer at high speed Stir; add 2mL dilute nitric acid drop by drop with a dropper during the above stirring process and continue to stir to make solution B; slowly pour B solution into A solution during the stirring process, and after stirring at high speed for 15min, a brown gel is formed, and then Leave to age in a water bath at a constant temperature of 60°C for 2 hours; dry in a drying oven at 110°C for 24 hours. Grind into large particles after dry...
Embodiment 2
[0043] Measure 140mL of absolute ethanol and pour it into a beaker, measure 30mL of butyl titanate and slowly pour it into absolute ethanol, and continue stirring at a medium speed on a magnetic stirrer; measure 3mL of glacial acetic acid with a dropper during the above stirring process Add drop by drop, and continue to stir to form a transparent solution A; weigh 4.5g of nickel nitrate hexahydrate, 0.93g of cerium nitrate hexahydrate, and 18g of magnesium nitrate hexahydrate, mix and dissolve in 20mL deionized water, and stir at high speed on a magnetic stirrer; During the above stirring process, add 2 mL of dilute nitric acid drop by drop with a dropper and continue stirring to form solution B; slowly pour solution B into solution A during the stirring process, and after stirring at high speed for 15 minutes, a brown gel is formed; Stand for aging at a constant temperature of 60°C for 2 hours; dry in a drying oven at 110°C for 24 hours. Grind into large particles after dryin...
Embodiment 3
[0047] Weigh 2.5g of nickel nitrate hexahydrate, 1.6g of cerium nitrate hexahydrate, 12.8g of magnesium nitrate hexahydrate, 32g of aluminum nitrate nonahydrate, mix and dissolve in 70mL deionized water, place on a magnetic stirrer and stir at high speed to form solution A; Take 22.2g of sodium hydroxide, dissolve it in 70mL of deionized water, and stir at high speed on a magnetic stirrer to form solution B; during the process of high-speed stirring, add solution B to solution A drop by drop with a dropper until the precipitation is complete. The precipitate was washed and filtered until the filtrate was neutral. The precipitate was dried in a drying oven at 110 °C for 24 h. Grind into large particles after drying; put the ground large particles into a muffle furnace for calcination at 700°C for 3 hours, and after the temperature drops to room temperature, take out the catalyst particles that are ground and sieved to the required particle size. The catalyst is called Ni-Ce-Mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com