Preparation method for dl-alpha tocopheryl acetate
A technology of tocopheryl acetate and low-level fatty acid ester, which is applied in the field of preparing dl-α tocopheryl acetate, can solve the problems of complex process route, complex process, high reaction temperature, etc., achieve good process stability, simple process, Good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 1.0 molar parts (194.5g) trimethylhydroquinone-1-ethyl ester in the reaction flask, then add solvent methyl acetate 500g, hydrobromic acid 0.05mol, ZnBr2 0.5mol, then add phytol 1.0 molar parts (297.0 g) After the feeding is completed, stir evenly, start heating, and start the reaction at a temperature of 40° C., and the reaction time is 4.0 hours, and the reaction is completed.
[0040] Add 100ml of water to the reaction solution, stir well, let stand, separate the water layer, repeat twice, the organic layer is orange-red, and the organic layer solvent is recovered under reduced pressure to obtain 461.5 g of the product dl-α tocopheryl acetate.
[0041] The water layers were combined, and the water was recovered under reduced pressure to obtain 151.4 g of the catalyst for mechanical use; the recovery of the above organic layer was 495 g of methyl acetate as a mechanical solvent.
[0042] For the detection of dl-α tocopheryl acetate samples, please refer to the ide...
Embodiment 2~11
[0045] According to the method described in Example 1, and according to the implementation objects and relevant parameters listed in Table 1 and Table 2, dl-alpha tocopheryl acetate was prepared.
[0046] Table 1
[0047]
[0048]
[0049] Table 2
[0050]
[0051]
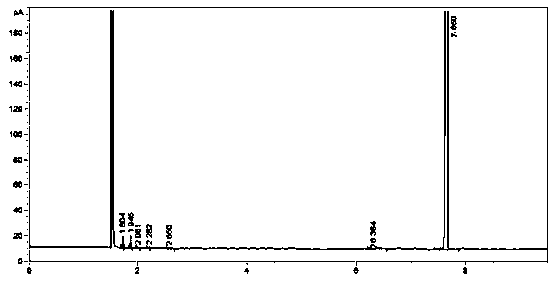
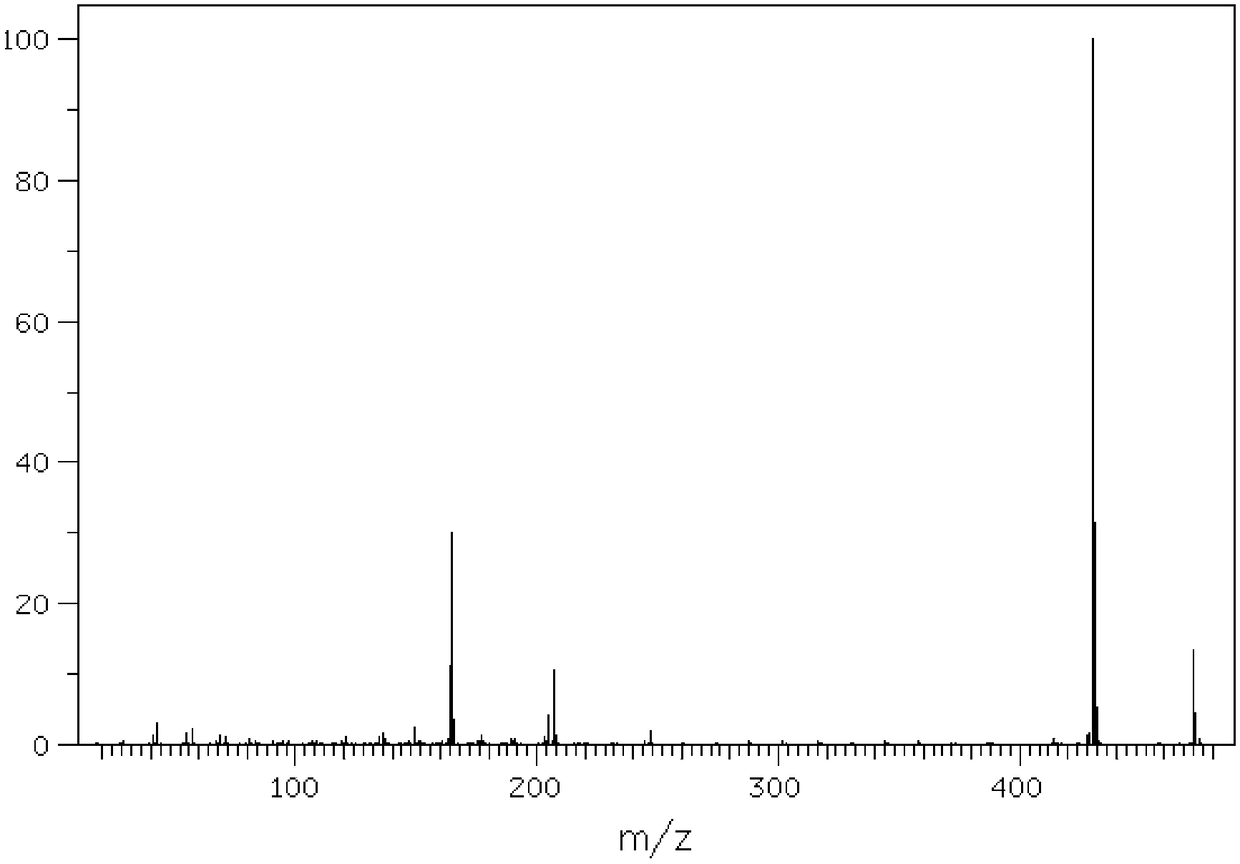
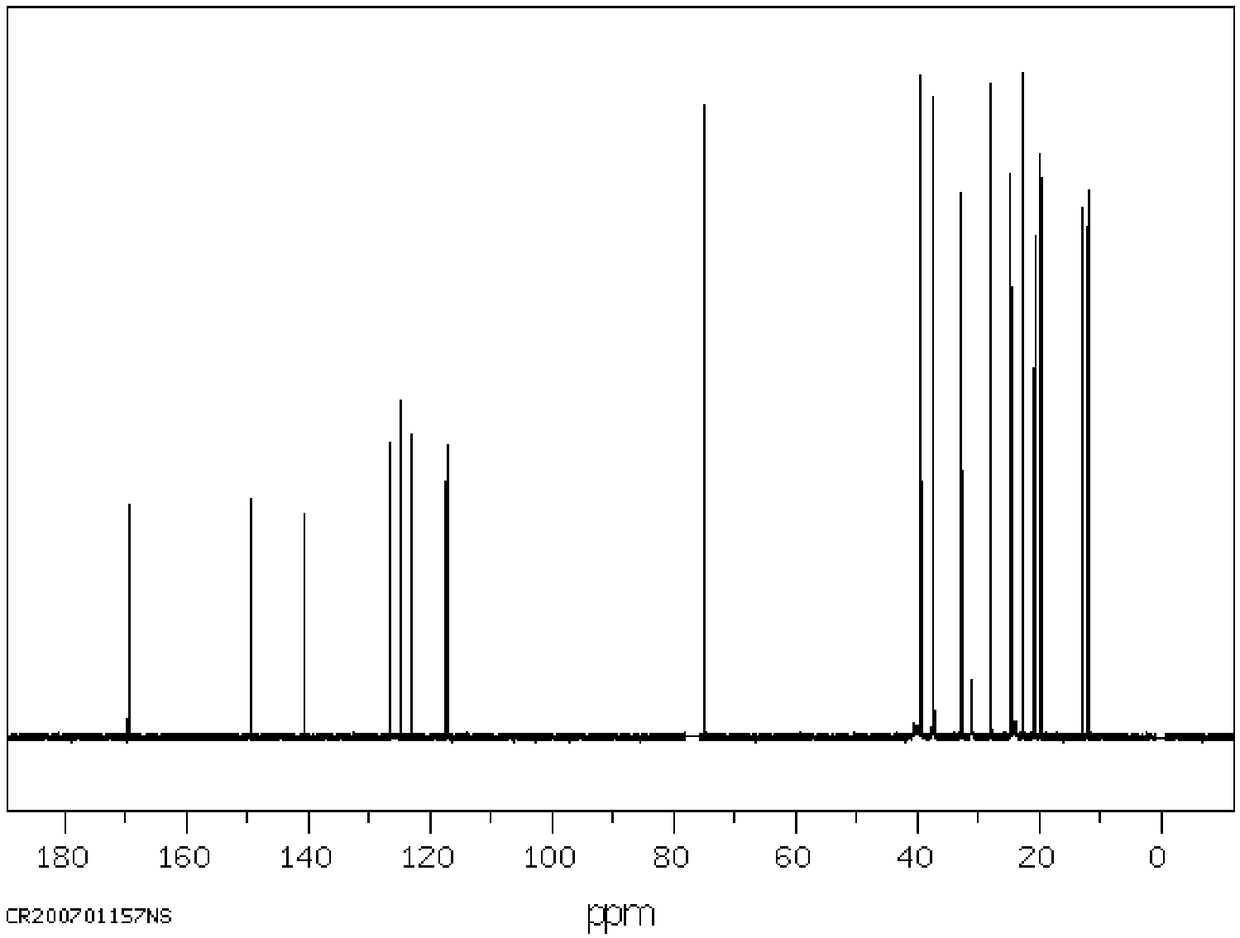
[0052] Detect the dl-alpha tocopheryl acetate samples of Examples 2-11, for the identification of the samples, please refer to Figure 1 to Figure 5 The chromatogram shows that the dl-alpha tocopherol content is 0.05%, the dl-alpha tocopherol acetate content is 96.90%, and the process experiment yield is 94.60% (total yield).
[0053] The methyl acetate content of the solvent used for detection is 99.2%, the solvent decomposition is little, and the yield is 98.4%; the catalyst hydrobromic acid used for detection is 4.7%, the yield is 85%, the ZnBr2 content is 71.0%, the yield is 96%, and the recovery effect of the catalyst is better.
Embodiment 12
[0055] In the reaction flask, add 1.0 mole parts (194.5g) trimethylhydroquinone-1-ethyl ester, then add the solvent ethyl acetate 695g, the catalyst 36.5g (containing hydrochloric acid 8%, AlCl content 69.5% ), and then add 1.0 molar parts of isophytol (297.0g), after the feeding is completed, stir evenly, start heating, and start the reaction at a temperature of 60° C., and the reaction time is 5.0 hours, and the reaction is completed.
[0056] Add 100ml of water to the reaction solution, stir well, let stand, separate the water layer, repeat 2 times, the organic layer is orange-red, and the organic layer solvent is recovered under reduced pressure to obtain 463.6 g of the product dl-α tocopheryl acetate.
[0057] The water layers were combined, and the water was recovered under reduced pressure to obtain 34.7 g of a catalyst for mechanical use; the recovery of the above organic layer was 692 g of ethyl acetate, a mechanical solvent.
[0058] For the detection of dl-α tocophe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com