Preparation method of small molecular proteins or polypeptides and fusion protein
A technology of small molecular proteins and fusion proteins, which is applied in the direction of fusion polypeptides, chemical instruments and methods, animal/human proteins, etc., can solve the problems of long production cycle, complicated process, and inability to make medicines, and achieve low cost, simple extraction methods, High safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Construction of embodiment 1 fusion partner expression plasmid
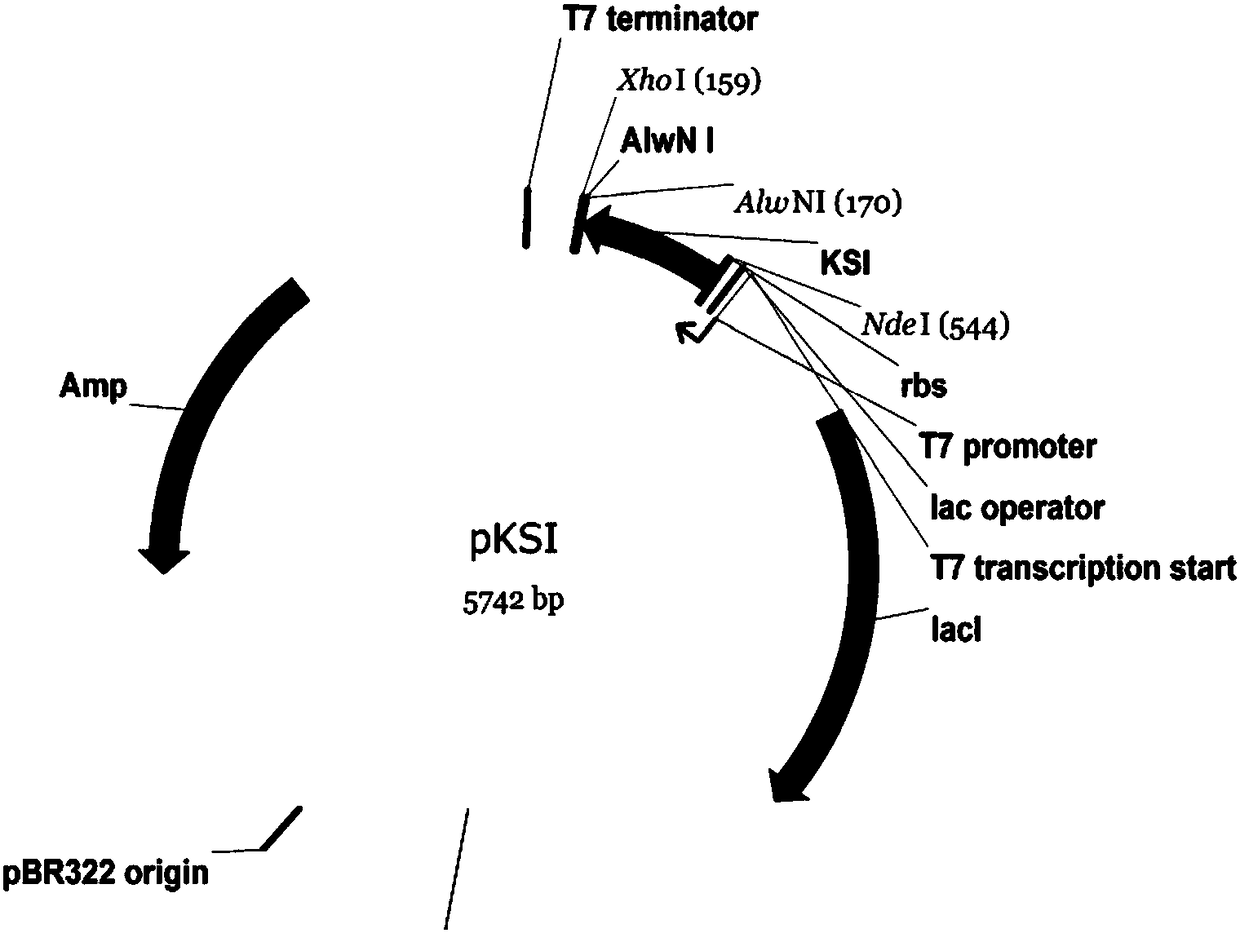
[0050] The expression plasmid contains a T7lac-type promoter and a T7 terminator, which can shut down expression when there is no inducer, and start expression when there is an inducer. The plasmid also contains a selectable marker for maintaining the stability of the gene of interest in E. coli.
[0051] Using aldosterone isomerase (KSI) as the fusion partner protein, the gene sequence encoding the fusion partner was artificially synthesized, and the start codon ATG was incorporated into its 5' end. In order to facilitate the cloning of different target protein molecules, the start codon was mixed A restriction enzyme NdeI site was incorporated, a restriction enzyme AlwNI site was incorporated at the 3' end, followed by an XhoI site. Synthetic DNA was digested with NdeI and XhoI, and ligated with the digested NdeI-XhoI fragment of the T7lac-type expression plasmid. The above-mentioned plasmids prolifera...
Embodiment 2
[0053] Example 2 Construction of Escherichia coli Expression System and Production of Human Insulin
[0054]Add a suitable connecting peptide to the amino terminal of the human insulin precursor molecule, and obtain the coding gene by artificial synthesis, specifically L-B(1-29)-AAK-A(1-21), L-B(1-30)-KWK - A (1-21), the 3' end of which incorporates a stop codon TAA, a restriction enzyme XhoI site is incorporated after the stop codon, and a restriction enzyme AlwNI site is incorporated at the 5' end. Synthetic DNA was digested with XhoI and AlwNI, and ligated with the digested XhoI-AlwNI fragment of pKSI plasmid. The ligation product was introduced into Escherichia coli, and multiplied in the presence of ampicillin, and the plasmid was isolated by conventional methods. Examination by a suitable restriction nuclease (e.g. XhoI, AlwNI, NdeI) shows that the plasmid DNA contains an inserted sequence, and sequence analysis shows that the plasmid DNA contains the human insulin prec...
Embodiment 3
[0061] Example 3 Construction of Escherichia coli Expression System and Fusion Protein KSI-L-Arg 34 Expression of GLP-1(7-37)
[0062] Add a suitable connecting peptide to the amino terminal of the GLP-1 analog precursor molecule, and obtain the coding gene by artificial synthesis, specifically L-Arg 34 GLP-1 (7-37), the 3' end incorporates the stop codon TAATGA, the restriction enzyme XhoI site is incorporated after the stop codon, and the restriction enzyme AlwNI site is incorporated at the 5' end. Synthetic DNA was digested with XhoI and AlwNI, and ligated with the digested XhoI-AlwNI fragment of plasmid pKSI and plasmid pmKSI. The ligation product was introduced into Escherichia coli, and multiplied in the presence of ampicillin, and the plasmid was isolated by conventional methods. Examination by a suitable restriction nuclease (e.g. XhoI, AlwNI, NdeI) shows that the plasmid DNA contains the inserted sequence, and sequence analysis shows that the plasmid DNA contains th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com