Preparation method and application of marine fungi aspergillus terreus butyrolactone compound butyrolactone-I
A technology of ester compounds and marine fungi, applied in the field of microorganisms, can solve the problems of high price, toxic and side effects, etc., and achieve the effects of simple method, increased yield and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
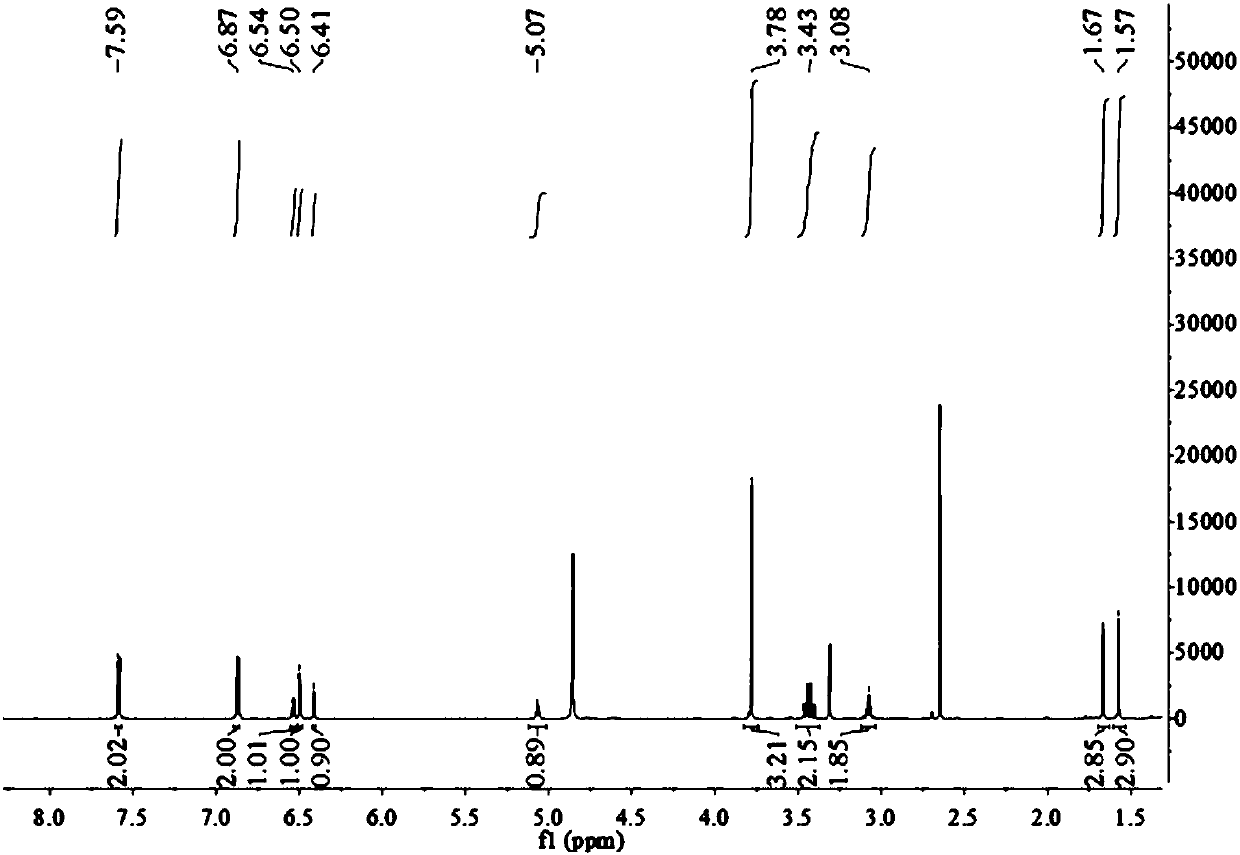
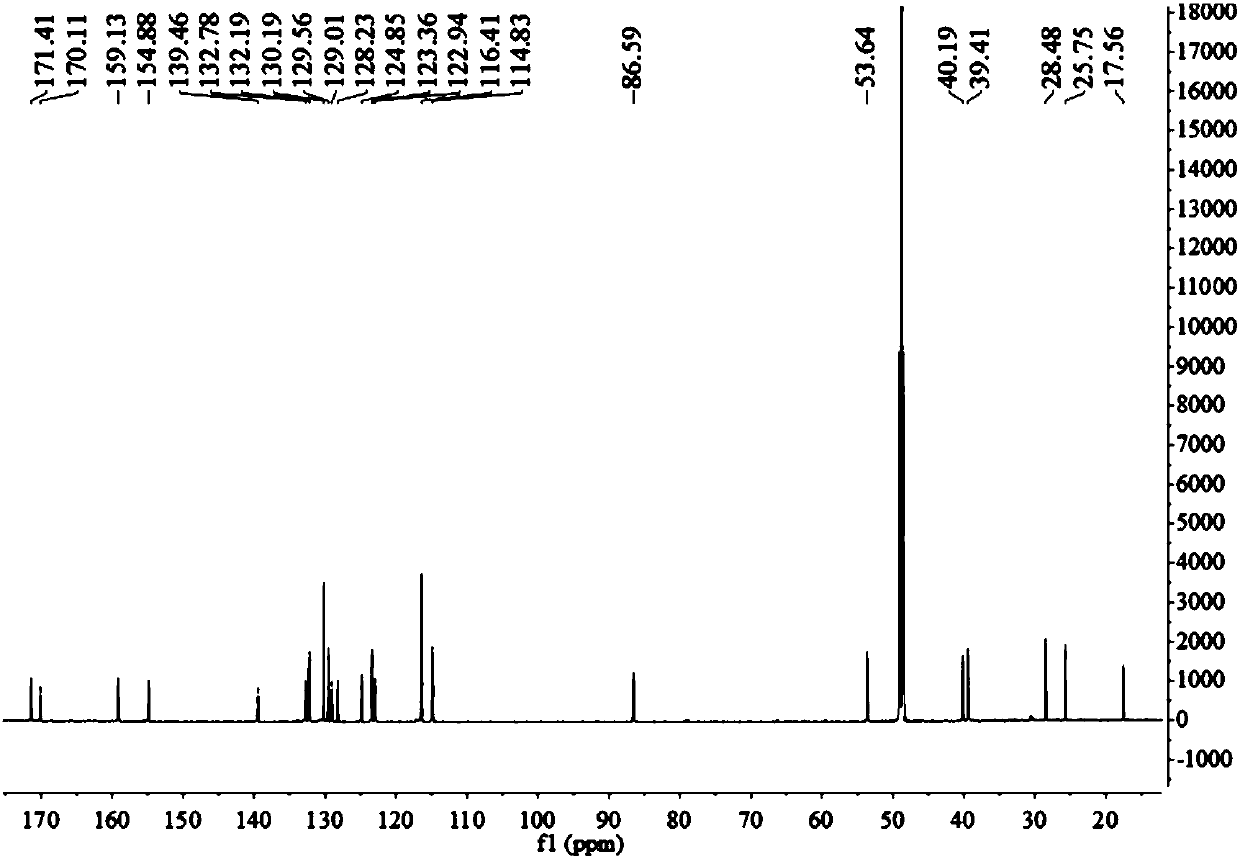
[0048] Example 1 The preparation method of marine fungus Aspergillus terreus C23-3 butyrolactone compound butyrolactone-I
[0049] The present embodiment provides a kind of preparation method of butyrolactone-I, specifically as follows:
[0050] Fermentation: Aspergillus terreus ( Aspergillus terreus ) GDMCC No.60316 was inoculated in the fungal liquid culture medium and left to ferment for 18-25 days, filtered, and the mycelia and fermentation broth were collected respectively;
[0051]Wherein, the formula of described fungal liquid culture medium is:
[0052] Fresh potato juice 400-600mL, sea salt 15-25g, sucrose 15-25g, peptone 3-8g, distilled water 400-600mL, pH 6-8.
[0053] 2. Crude extraction: the fermentation broth obtained in step 1 was ultrasonically extracted 3 times with ethyl acetate and concentrated, the mycelium obtained in step 1 was ultrasonically extracted with methanol and concentrated under reduced pressure, and the obtained concentrates were combined to...
Embodiment 2
[0069] The free radical scavenging experiment of embodiment 2 butyrolactone-I
[0070] (1) Test method
[0071] DPPH free radical scavenging activity test: Vc was used as a positive control. Experimental group (A 1 ): Add different concentrations of monomeric compound NYY-1 (0.05-100 μM) in turn to a 96-well plate, dry it, add 50 μL each of DMSO and 0.16 mM DPPH solution in turn; the control group (A 2 ): use 50 μL methanol instead of 0.16 mM DPPH solution, the others are the same as the experimental group; blank group (A 3 ): no sample, other same as experimental group; blank control group (A 4 ): without adding samples, the others are the same as the control group. React in the dark for 30 min, and measure its absorbance at a wavelength of 517 nm. Use the origin software to make a clearance-concentration semi-logarithmic curve, and calculate its half-clearance concentration (EC 50 )value.
[0072]
[0073] ABTS free radical scavenging activity test: using the total...
Embodiment 3
[0079] Example 3 Determination of intracellular antioxidant and anti-inflammatory activities of butyrolactone-I
[0080] (1) Test method
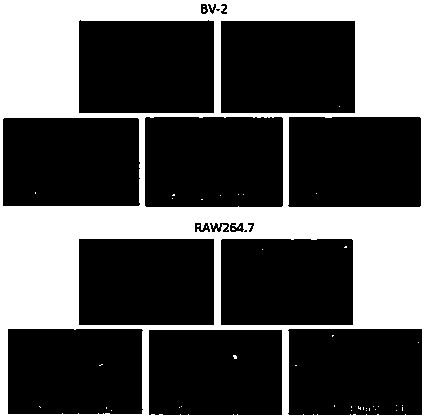
[0081] Mouse microglial cells BV2 and macrophages RAW267.4 with good logarithmic phase growth were randomly divided into blank control group, LPS group, butyrolactone compound pretreatment+LPS group. The autotoxicity of butyrolactone-I to BV2 and RAW264.7 cells was evaluated by MTT method, the inhibitory effect of butyrolactone I on LPS-induced intracellular NO in BV2 and RAW264.7 cells was determined by Gress method, and the DCFH-DA fluorescent probe was used to detect butyrolactone-I. Inhibitory effect of lactone-I on LPS-induced ROS in BV-2 and RAW264.7 cells. Western blot was used to determine the effect of butyrolactone-I on the expression of iNOS and COX-2 proteins in RAW264.7 cells.
[0082] (2) Results
[0083] Such as Figure 3~Figure 5 As shown, the butyrolactone compound butyrolactone-I can protect cells from oxidative damage ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com