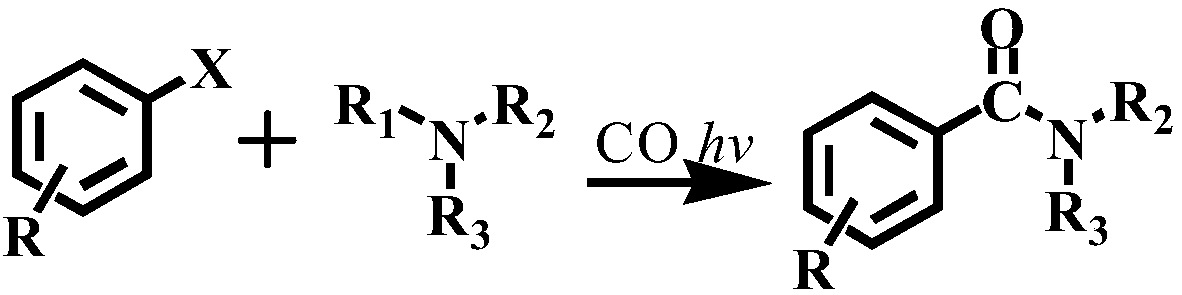Method for preparing amide through carbon monoxide-participating carbonyl reaction
A technology of carbonylation reaction and carbon monoxide, which is applied in the preparation of organic compounds, chemical instruments and methods, and the formation/introduction of amide groups, etc., can solve the problems of difficult purification of products, and achieve simple purification, no catalyst addition, and reaction The effect of shortening the route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 (the mode of operation and the amount of substrate used in all later implementation cases are consistent with Case 1)
[0025] 1. Solvent and substrate purification
[0026] Use a vacuum distillation device to remove water from the reaction solvent and the substrate, and then place the dehydrated halogenated aromatic hydrocarbons and organic amines in an inert gas glove box for subsequent use.
[0027] 2. Configuration of the reaction system in a dry atmosphere
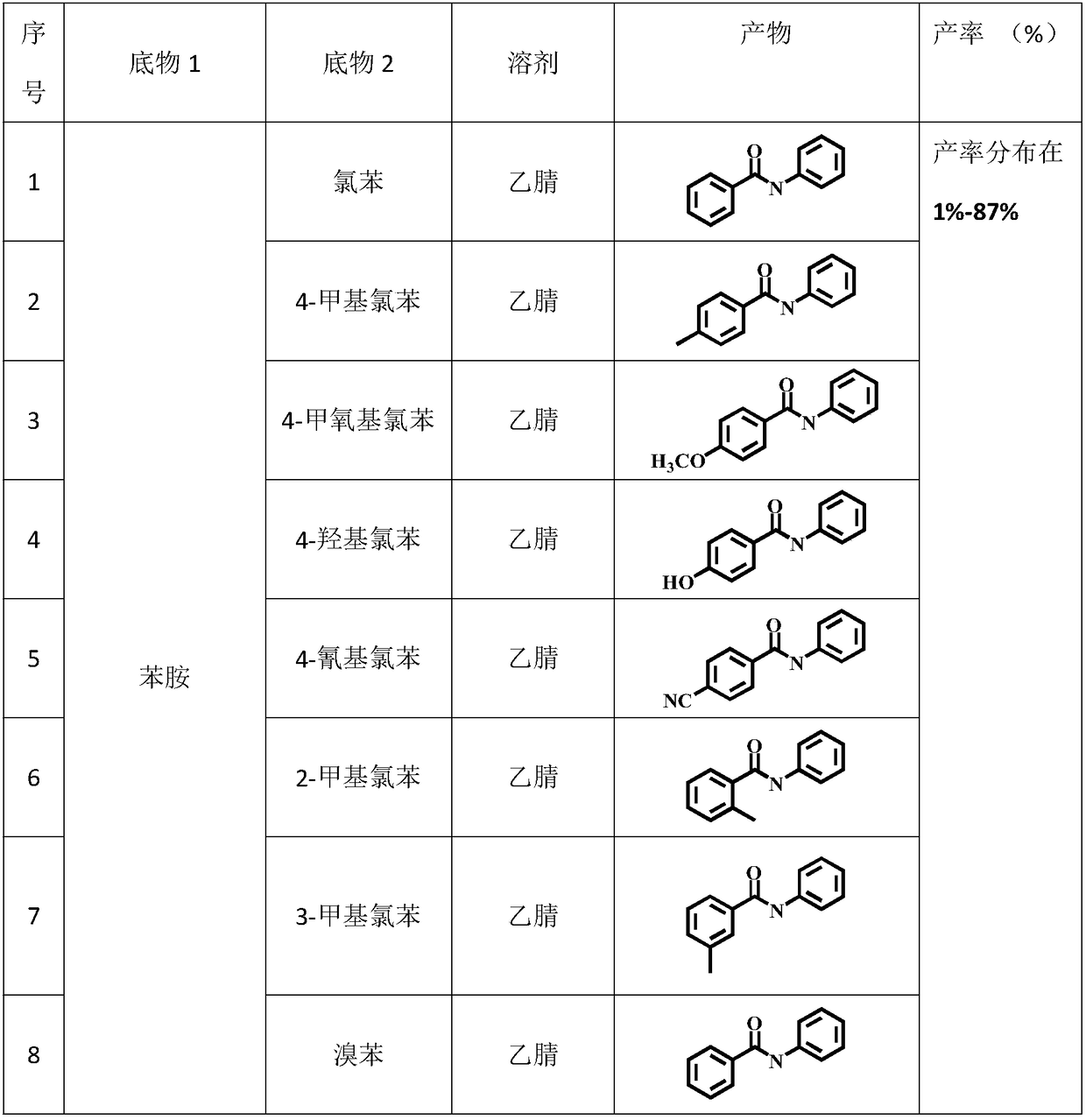
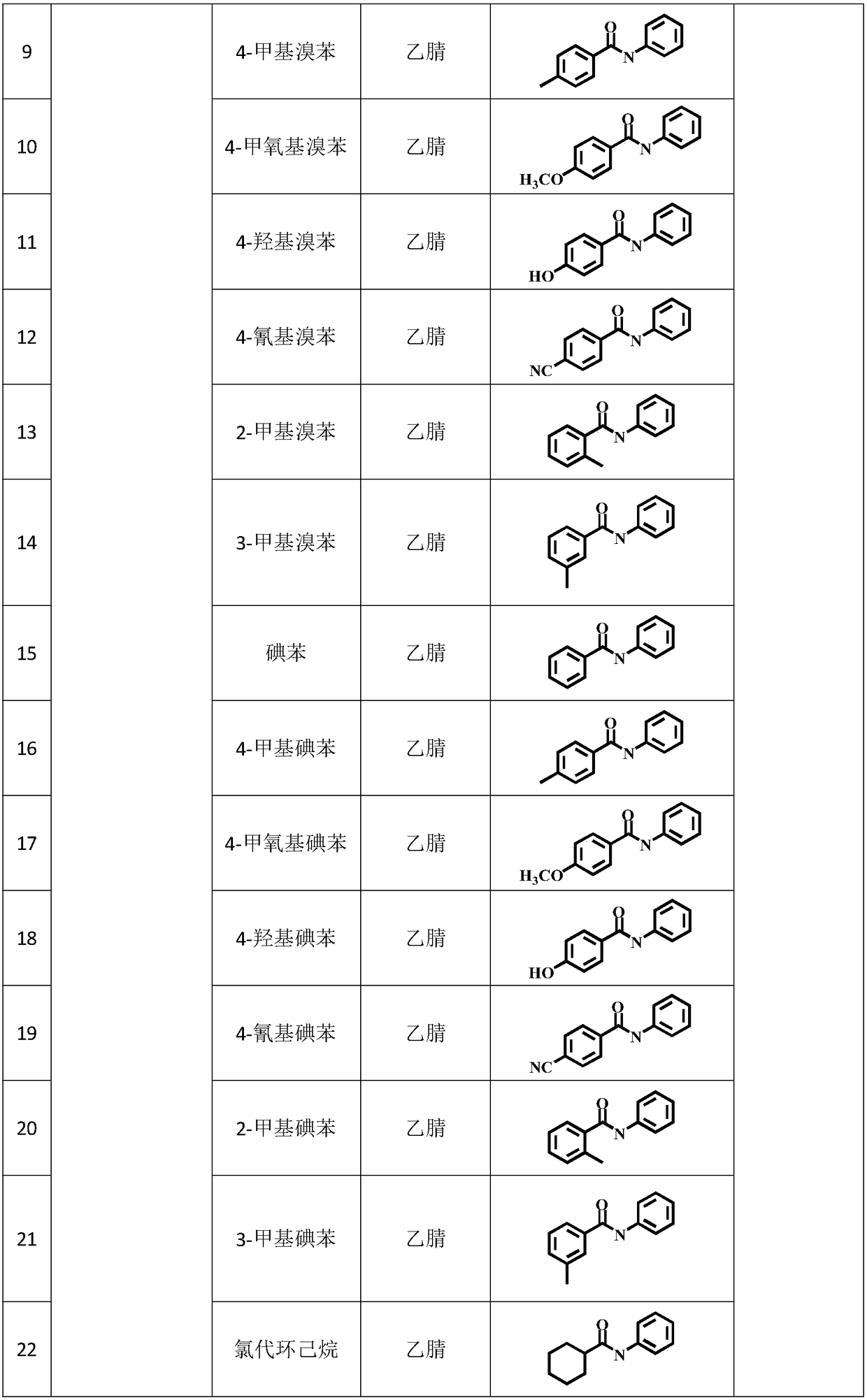
[0028] Move the dried quartz reactor into the glove box, put the magnet into the reactor, transfer 1mL of acetonitrile, 1-2mL of chlorobenzene, and 0.1-0.3mmol of aniline with a pipette gun, seal the reactor with a parafilm, and then Remove from glove box.
[0029] 3. Reaction pretreatment
[0030] Carbon monoxide is injected into the reaction system by bubbling and maintained for 10-15 minutes, the purpose is to discharge the oxygen in the reaction system. Afterwards, carbon monoxide with a purit...
Embodiment 2
[0034] The specific organic amine synthesis method is basically the same as in Example 1 of this part, except that chlorobenzene is changed to 4-methylchlorobenzene.
Embodiment 3
[0036] Concrete organic amine synthesis method is basically the same as this part embodiment 1, and difference is that chlorobenzene is changed into 4-methoxychlorobenzene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com