Organic electroluminescence compound and application thereof as well as organic electroluminescence device
A compound and luminescent technology, applied in the field of optoelectronic technology materials, can solve problems affecting the physical process of devices, differences in physical properties, poor film morphology, etc., and achieve the effects of simplifying the preparation procedure, improving the service life, and increasing the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
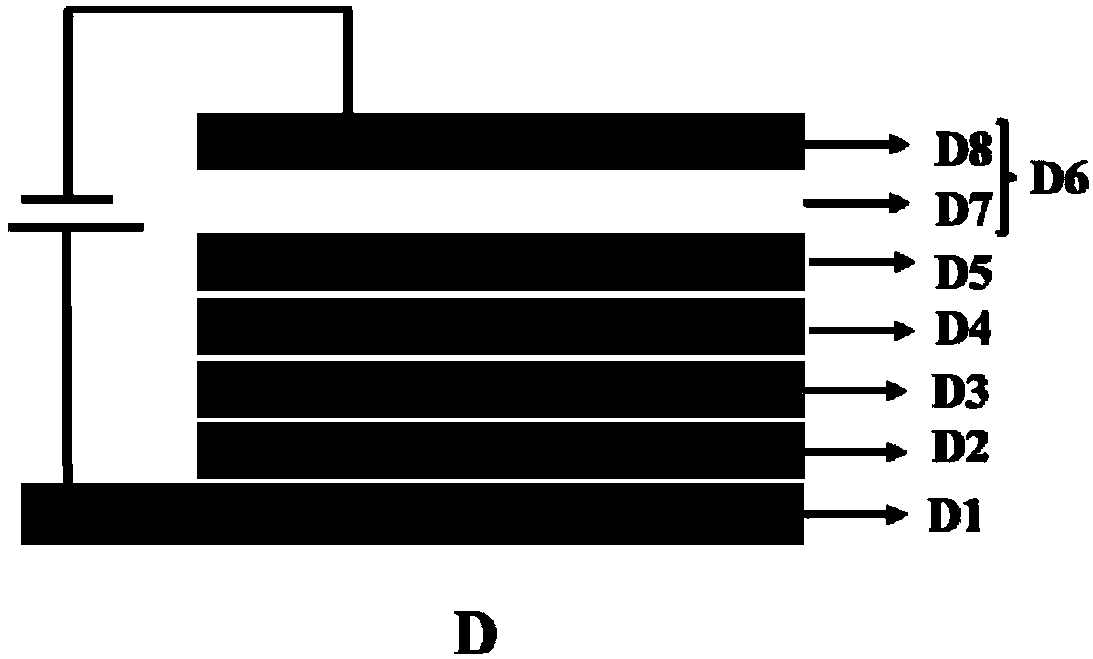
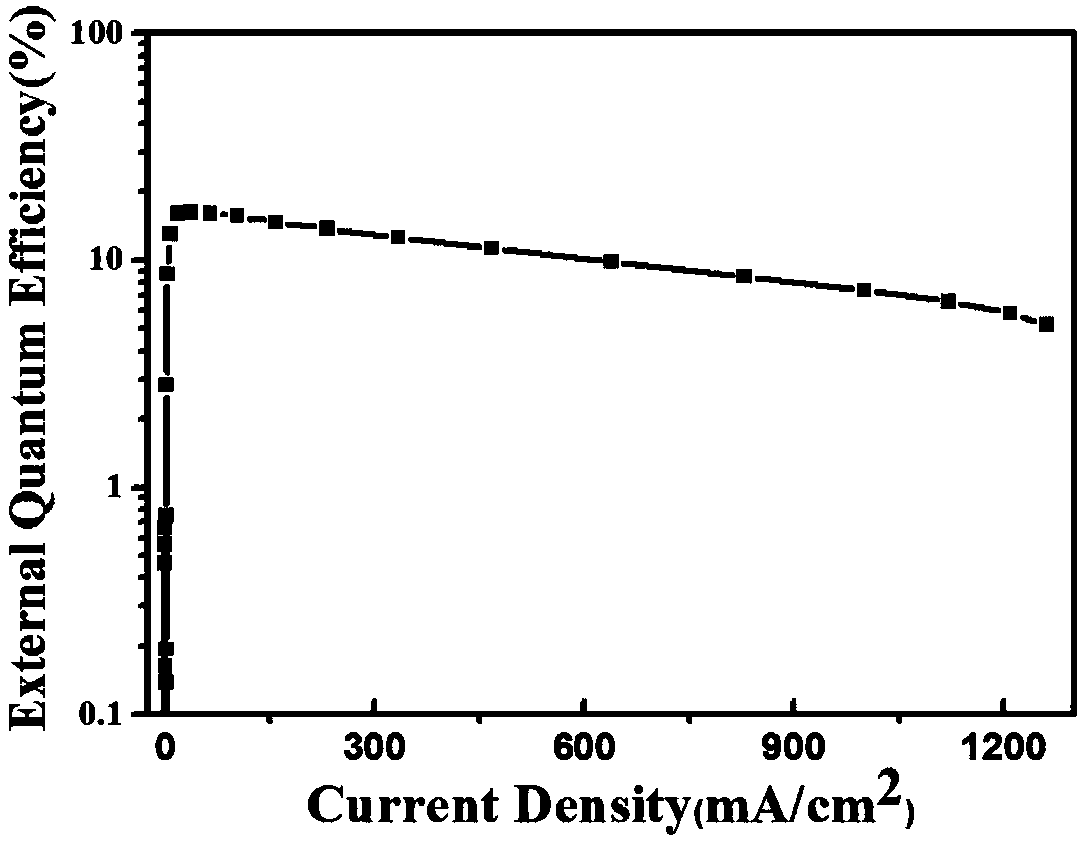
Image
Examples
Embodiment 1
[0056] The structural formula is Synthesis of the first target compound:
[0057] 5-bromoisophenyldialdehyde (2.11g, 10mmol) and N 1 -Phenylbenzene-1,2-diamine (4.41g, 24mmol) was added to the 2 S 2 o 5 (3.32g 20mmol) in dimethylformamide (DMF) solution, reacted at 90°C for 48h under the protection of inert gas. DMF was distilled off under reduced pressure, and the obtained residue was purified by column chromatography to obtain the intermediate product BPBI (3.7 g, yield 68.5%). The intermediate product is identified and analyzed by a mass spectrometer, and the result is: mass spectrum (EI): m / z C 32 h 21 BrN 4 Theoretical value: 541.45; measured value: 541(M) + .
[0058] 3-Bromopyrrole (1.449g, 10mmol), 4-(2-hydroxyethoxy)benzoyl (0.83g, 5mmol) and BF3 (0.34, 5mmol) were added to 100mL DMF, at 110°C and inert gas protection Under reaction 48h. DMF was distilled off under reduced pressure, and the obtained residue was purified by column chromatography to obtain t...
Embodiment 2
[0062] The structural formula is Synthesis of the second target compound:
[0063] DCzDMAC (3.85g, 5.04mmol), compound A-BPBI (2.0g, 2.1mmol), CuI (0.19g, 1.0mmol) and K 3 PO 4 (1.06g, 8mmol) was added to 100mL toluene, and after degassing with argon for 30 minutes, trans-1,2-cyclohexanediamine (0.245mL, 2.0mmol) was added, and the reaction mixture was reacted under reflux for 48 Hour. After removing the solvent, the residue was extracted three times with dichloromethane (3×100mL), the organic phases were combined and dried over anhydrous magnesium sulfate, and the residue obtained by concentration was purified by column chromatography using dichloromethane: n-hexane as eluent to obtain The product DFBDCz (3.46 g, 71.4% yield). The intermediate product is identified and analyzed by a mass spectrometer, and the result is: mass spectrometry mass spectrometry (MALDI-TOF): m / z C 159 h 153 BF 2 N 12 o 2 Theoretical value: 2312.2; measured value: 2312(M) + .
Embodiment 3
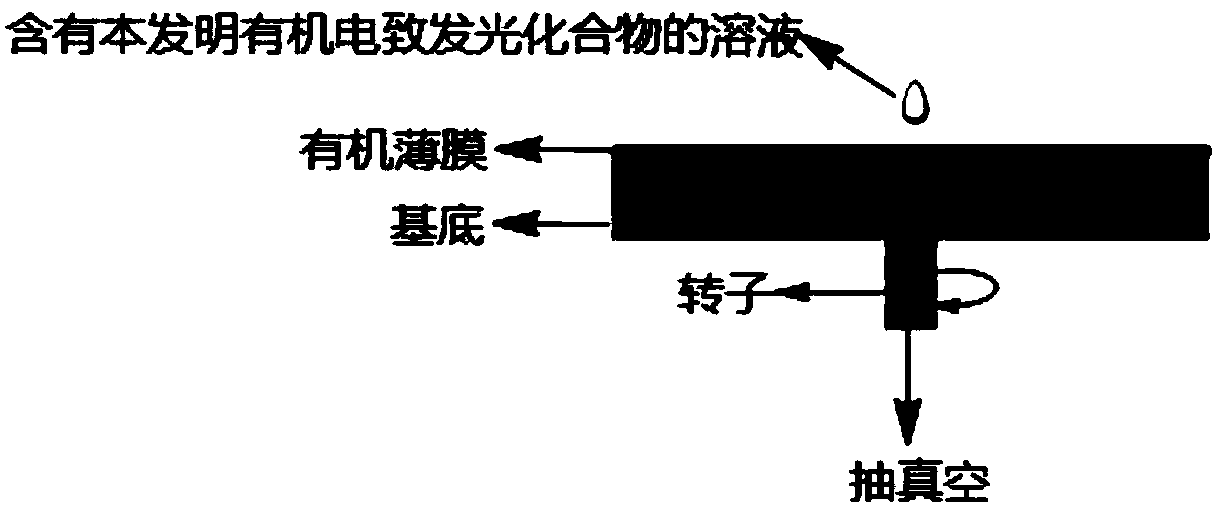
[0065] The first target compound, DFBDCz, was dissolved in chlorobenzene to prepare a solution with a mass concentration of 15 mg / mL. like figure 1 As shown, 40 μL of the solution was dropped onto an indium tin oxide glass substrate, and an organic thin film was prepared on a table top coater (KW-4A type) with a rotation speed of 1500 r / min and a spin coating time of 30 s. The measured thickness of the obtained organic thin film was 40 nm, and the root mean square roughness (Rq) was 0.76 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com